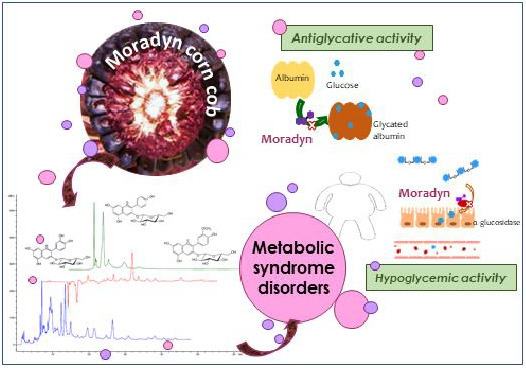
A New Italian Purple Corn Variety (Moradyn) Byproduct Extract: Antiglycative and Hypoglycemic In Vitro Activities and Preliminary Bioaccessibility Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Moradyn Cob Extract by RP-HPLC-DAD-ESI-MSn
2.2. Evaluation of Hypoglycemic Activity
2.3. Evaluation of Antiglycative Effect Using In Vitro Bovine Serum Albumin-Methylglyoxal, -Glucose, -Fructose, and -Ribose Systems
2.4. Bioaccessibility Study
3. Materials and Methods
3.1. Reagents
3.2. Moradyn Cob Extract Preparation
3.3. Anthocyanin Fraction Purification
3.4. RP-HPLC-DAD-ESI-MSn Analysis
3.5. Evaluation of Hypoglycemic Activity
3.5.1. α-Amylase Inhibitory Activity
3.5.2. α-Glucosidase Inhibitory Activity
3.6. Evaluation of Antiglycative Capacity
3.7. In Vitro Digestion Procedure
3.8. Bioaccessibility Evaluation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lao, F.; Giusti, M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Met. 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Yonemaru, J.; Miki, K.; Choi, S.; Kiyosawa, A.; Goto, K. A genomic region harboring the Pl1 allele from the Peruvian cultivar JC072A confers purple cob on Japanese flint corn (Zea mays L.). Breed. Sci. 2018, 68, 582–586. [Google Scholar] [CrossRef] [Green Version]
- Chatham, L.A.; West, L.; Berhow, M.A.; Vermillion, K.E.; Juvik, J.A. Unique flavanol-anthocyanin condensed forms in Apache red purple corn. J. Agric. Food Chem. 2018, 66, 10844–10854. [Google Scholar] [CrossRef] [PubMed]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic variation in anthocyanins, phenolic compounds, and antioxidant activity in cob and husk of purple field corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Kim, H.W.; Won, S.R.; Min, H.; Park, K.J.; Park, J.Y.; Ahn, M.S.; Rhee, H.I. Corn husk as a potential source of anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Somavat, P.; Singh, V.; Chatham, L.; Gonzalez de Mejia, E. A comparative study of anthocyanin distribution in purple and blue corn coproducts from three conventional fractionation processes. Food Chem. 2017, 231, 332–339. [Google Scholar] [CrossRef]
- Jing, P.; Giusti, M. Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk. J. Agric. Food Chem. 2005, 53, 8775–8781. [Google Scholar] [CrossRef]
- Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J. LC–MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoǧlu, G.; Gökmen, V.; Vančetovic, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Fiore, A.; Fogliano, V.; Morales, F.J. Carbonyl trapping and antiglycative activities of olive oil mill wastewater. Food Funct. 2015, 6, 574–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sri Harsha, P.S.C.; Lavelli, V.; Scarafoni, A. Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem. 2014, 156, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Morales, F.J. Evaluation of an olive leaf extract as a natural source of antiglycative compounds. Food Res. Int. 2017, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Kuhla, A.; Ludwig, S.C.; Kuhla, B.; Münch, G.; Vollmar, B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiol. Aging 2015, 36, 753–761. [Google Scholar] [CrossRef]
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J.A. Methylglyoxal-derived advanced glycation endproducts in multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Somavat, P.; Singh, V.; Gonzalez de Mejia, E. Chemical characterization of proanthocyanidins in purple, blue, and red maize coproducts from different milling processes and their antiinflammatory properties. Ind. Crops Prod. 2017, 109, 464–475. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; MunõZ, A.M.; Alvarado-OrtìZ, C.; Alvarado, A.; Yánez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilavech, T.; Ngamukote, S.; Abeywardena, M.; Adisakwattana, S. Protective effects of cyanidin-3-rutinoside against monosaccharides-induced protein glycation and oxidation. Int. J. Biol. Macromol. 2015, 75, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Chaiittianana, R.; Sutthanutb, K.; Rattanathongkomc, A. Purple corn silk: A potential anti-obesity agent with inhibition on adipogenesis and induction on lipolysis and apoptosis in adipocytes. J. Ethnopharmacol. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Guzmán-Gerόnimo, R.I.; Alarcόn-Zavaleta, T.M.; Oliart-Ros, R.M.; Meza-Alvarado, J.E.; Herrera-Meza, S.; Chávez-Servia, J.L. Blue maize extract improves blood pressure, lipid profiles, and adipose tissue in high-sucrose diet-induced metabolic syndrome in rats. J. Med. Food 2017, 20, 110–115. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from purple corn ameliorated TNF-α-induced inflammation and insulin resistance in 3T3-L1 adipocytes via activation of insulin signaling and enhanced GLUT4 translocation. Mol. Nutr. Food Res. 2017, 61, 1700362–1700375. [Google Scholar] [CrossRef]
- Lago, C.; Cassani, E.; Zanzi, C.; Landoni, M.; Trovato, R.; Pilu, R. Development and study of a maize cultivar rich in anthocyanins: Coloured polenta, a new functional food. Plant Breed. 2014, 113, 210–217. [Google Scholar] [CrossRef]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Morales-Ortega, A.; Carvajal-Millan, E.; Brown-Bojorquez, F.; Rascón-Chu, A.; Patricia Torres-Chavez, P.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Martínez-López, A.L.; Campa-Mada, A.C. Entrapment of probiotics in water extractable arabinoxylan gels: Rheological and microstructural characterization. Molecules 2014, 19, 3628–3637. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The nutraceutical bioavailability classification scheme: Classifying nutraceuticals according to factors limiting their oral bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Lima, K.; Silva, O.; Figueira, M.E.; Piresa, C.; Cruza, D.; Gomes, S.; Muchagato, E.M.; Duartea, M.P. Influence of the in vitro gastrointestinal digestion on the antioxidant activity of Artemisia gorgonum Webb and Hyptis pectinata (L.) Poit. infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zeng, J.; Zhai, L.; Liu, Y.; Wu, H.; Zhang, R.; Li, Z.; Xia, E. Effect of in vitro simulated gastrointestinal digestion on polyphenol and polysaccharide content and their biological activities among 22 fruit juices. Food Res. Int. 2017, 102, 156–162. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Pérez-Alonso, J.; Salinas-Moreno, Y.; Mateus, N.; Silva, A.M.S.; De Freitas, V.; Santos-Buelg, C. Flavanol-anthocyanin pigments in corn: NMR characterisation and presence in different purple corn varieties. J. Food Compos. Anal. 2008, 21, 521–526. [Google Scholar] [CrossRef]
- Lu, L.; Song, F.-R.; Tsao, R.; Jin, Y.-R.; Liu, Z.-Q.; Liu, S.-Y. Studies on the hemolytic and heterolytic cleavage of kaempferol and kaempferide glycosides using electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; He, Q.; Song, Y.; Qu, H.; Cheng, Y. Characterization and identification of isomeric flavonoid O-diglycosides from genus Citrus in negative electrospray ionization by ion trap mass spectrometry and time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 598, 110–118. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three Citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, N.; Kaur, A. Characteristics of white, yellow, purple corn accessions: Phenolic profile, textural, rheological properties and muffin making potential. J. Food Sci. Technol. 2018, 55, 2334–2343. [Google Scholar] [CrossRef]
- Gálvez Ranilla, L.; Christopher, A.; Sarkar, D.; Shetty, K.; Chirinos, R.; Campos, D. Phenolic composition and evaluation of the antimicrobial activity of free and bound phenolic fractions from a Peruvian purple corn (Zea mays L.) accession. J. Food Sci. 2017, 82, 2968–2976. [Google Scholar] [CrossRef]
- Milella, L.; Milazzo, S.; De Leo, M.; Vera Saltos, M.B.; Faraone, I.; Tuccinardi, T.; Lapillo, M.; De Tommasi, N.; Braca, A. α-Glucosidase and α-amylase Inhibitors from Arcytophyllum thymifolium. J. Nat. Prod. 2016, 79, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Antioxidant and enzyme inhibitory activities of blueberry anthocyanins prepared using different solvents. J. Agric. Food Chem. 2013, 61, 4441–4447. [Google Scholar] [CrossRef]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Thilavech, T.; Abeywardenab, M.Y.; Adams, M.; Dallimore, J.; Adisakwattana, S. Naturally occurring anthocyanin cyanidin-3-rutinoside possesses inherent vasorelaxant actions and prevents methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed. Biomed. Pharmacother. 2015, 95, 1251–1259. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef] [Green Version]
- Suantawee, T.; Cheng, H.; Adisakwattana, S. Protective effect of cyanidin against glucose- and methylglyoxal-induced protein glycation and oxidative DNA damage. Int. J. Biol. Macromol. 2016, 93, 814–821. [Google Scholar] [CrossRef]
- Sadowska-Barttosz, I.; Galiniak, S.; Bartosz, G. Kinetics of glycoxidation of bovin serum albumin by glucose, fructose and ribose and its prevention by food components. Molecules 2014, 19, 18828–18849. [Google Scholar] [CrossRef] [Green Version]
- Justino, A.B.; Pereira, M.N.; Vilela, D.D.; Peixoto, L.G.; Martins, M.M.; Teixeira, R.R.; Miranda, N.C.; Da Silva, N.M.; De Sousa, R.M.F.; De Oliveira, A.; et al. Peel of raticum fruit (Annona crassiflora Mart.) as a source of antioxidant compounds with a-amylase, a-glucosidase and glycation inhibitory activities. Bioorg. Chem. 2016, 69, 167–182. [Google Scholar] [CrossRef]
- Sri Harsha, P.S.C.; Gardana, C.; Simonetti, P.; Spigno, G.; Lavelli, V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Biores. Technol. 2013, 140, 263–268. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Gao, F.; Shan, F.; Bian, J.; Zhao, C. Study on the Interaction between 3 Flavonoid Compounds and α-Amylase by Fluorescence Spectroscopy and Enzymatic Kinetics. J. Foos Sci. 2009, 74, 199–203. [Google Scholar] [CrossRef]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of Selected Phenolic Compounds to Proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Haitian Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Ni, X.; Yang, F.; Chen, X. Interaction of natural polyphenols with a-amylase in vitro: Molecularproperty–affinity relationship aspect. Mol. BioSyst. 2011, 7, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Spínola, V.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Molina-García, L.; Castilho, P.C. Polyphenolic profile and antioxidant activities of Madeiran elderberry (Sambucus lanceolata) as affected by simulated in vitro digestion. Food Res. Int. 2017, 100, 404–410. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Food Funct. 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Gardeli, C.; Varela, K.; Krokida, E.; Mallouchos, A. Investigation of anthocyanins stability from pomegranate juice (Punica granatum L. cv Ermioni) under a simulated digestion process. Medicines 2019, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.C.; Chen, C.L.; Jeng, T.L.; Linc, T.C.; Sung, J.M. Bioavailability of cranberry bean hydroalcoholic extract and its inhibitory effect against starch hydrolysis following in vitro gastrointestinal digestion. Food Res. Int. 2014, 64, 939–945. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; Macedo, D.; McDougall, G.J.; LeitãO, M.C.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effect of blackberry (Rubus sp.) polyphenols is potentiated after simulated gastrointestinal digestion. Food Chem. 2012, 131, 1443–1452. [Google Scholar] [CrossRef]
- Scorrano, S.; Lazzoi, M.R.; Mergola, L.; Di Bello, M.P.; Del Sole, R.; Vasapollo, G. Anthocyanins profile by Q-TOF LC/MS in Myrtus communis berries from Salento Area. Food Anal. Met. 2017, 10, 2404–2411. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Corana, F.; Papetti, A. Cretan tea (Origanum dictamnus L.) as a functional beverage: An investigation on antiglycative and carbonyl trapping activities. Food Funct. 2017, 9, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Meeprom, A.; Cheng, H.; Adisakwattana, S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage in bovine serum albumin. Molecules 2013, 18, 13886–13903. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 7, 2544–2550. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Compound | Rt (min) | Precursor Ion (m/z) | HPLC-ESI-MSn m/z (% of Base Peak) | Compound Identity |
|---|---|---|---|---|
| 1a | 5.92 | 449 * | MS2[449]: 287(100) | cyanidin-3-O-glucoside |
| 2a | 6.41 | 433 * | MS2[433]: 271(100) | pelargonidin-3-O-glucoside |
| 3a | 6.53 | 431 | MS2[431]: 269 (100), 268 (85) | apigenin-7-O-glucoside |
| 4a | 8.23 | 463 * | MS2[463]: 301(100) | peonidin-3-O-glucoside |
| 5 | 8.49 | 641 | MS2[641]: 479(100), 317(27) MS3[479]: 317(100) | myricetin-3,7-di-O-hexoside |
| 6 | 8.70 | 459 | MS2[459]: 235(30), 193(100), 149(30) | ferulic acid derivative |
| 7 | 9.77 | 479 | MS2[479]: 317(100), 316(5), 299(70) | myricetin-7-O-hexoside |
| 8 | 10.67 | 367 | MS2[367]: 191(100), 173(20) MS3[191]: 172(40), 127(80), 85(100) | 5-O-feruloylquinic acid |
| 9 | 11.00 | 639 | MS2[639]: 477(100), 315(5) MS3[477]: 315(100), 300(10) | isorhamnetin-3,7-di-O-hexoside |
| 10 | 15.76 | 609 | MS2[609]: 463(5), 301(100), 300(50) | quercetin-7-O-p-cumaroylhexoside |
| 11a | 17.17 | 463 | MS2[463]: 301(100), 300(30) | quercetin-7-O-glucoside |
| 12 | 17.97 | 609 | MS2[609]: 447(100), 285(30) | keampferol-3,7-di-O-hexoside |
| 13 | 19.97 | 533 | MS2[533]: 447(26), 285(100), 284(38) MS3[285]: 267(15), 257(100), 241(25), 199(10), 163(5) | keampferol-7-O-(6”-O-malonyl)-hexoside |
| 14 | 20.66 | 593 | MS2[593]: 447(70), 285(100), 257(15) MS3[285]: 267(15), 257(100), 241(10), 199(20) | keampferol-7-O-rutinoside |
| 15 | 21.85 | 623 | MS2[623]: 477(20), 315(100) MS3[315]: 300(100) | isorhamnetin-7-O-rutinoside |
| 16 | 22.24 | 447 | MS2[447]: 327(20), 285(100), 284(85), 257(30), 255(5) | kaempferol-7-O-glucoside |
| 17 | 23.26 | 477 | MS2[477]: 315(35), 314(100) MS3[315]: 300(100) | isorhamnetin-3-O-hexoside |
| 18a | 27.52 | 447 | MS2[447]: 285(100), 284(95), 151(10), 133(6) MS3[285]: 267(40), 257(20), 241(40), 199(10), 175(100) | luteolin-7-O-glucoside |
| 19 | 33.87 | 785 | MS2[785]: 609(20), 447(100) MS3[447]: 285(100), 284(80) | kaempferol-3-O-hexosyl-7-O-glucuronilhexoside |
| 20 | 34.67 | 815 | MS2[815]: 639(60), 477(100) MS3[477]: 315(100), 300(10) | isorhamentin-3-O-hexosyl-7-O-glucuronilhexoside |
| Assay | CE α-Glucosidase Inhibitory Activity | AF α-Glucosidase Inhibitory Activity |
|---|---|---|
| BSA-MGO system | ||
| 1 day | 0.908 | 0.977 |
| 2 days | 0.840 | 0.971 |
| 3 days | 0.813 | 0.991 |
| 7 days | 0.800 | 0.993 |
| BSA-GLU system | ||
| 7 day | 0.802 | 0.885 |
| 14 days | 0.337 | 0.895 |
| 21 days | 0.488 | 0.970 |
| 28 days | 0.021 | 0.718 |
| BSA-FRU system | ||
| 1 day | 0.728 | 0.984 |
| 4 days | 0.745 | 0.987 |
| 7 days | 0.803 | 0.982 |
| 14 days | 0.915 | 0.983 |
| BSA-RIB system | ||
| 1 h | 0.826 | 0.918 |
| 3 h | 0.841 | 0.954 |
| 6 h | 0.827 | 0.935 |
| 24 h | 0.880 | 0.879 |
| Compound | Oral Phase | Gastric Phase | Duodenal Phase | Colon Phase |
|---|---|---|---|---|
| 1 | 63.96 ± 1.4 | 69.78 ± 2.60 | 100 | 100 |
| 2 | 57.86 ± 0.73 | 73.33 ± 1.18 | 100 | 100 |
| 4 | 74.94 ± 0.78 | 84.45 ± 1.94 | 100 | 100 |
| 5 | 61.04 ± 0.48 | 80.03 ± 0.24 | 100 | 100 |
| 6 | 100 | 100 | 100 | 100 |
| 7 | 54.97 ± 0.69 | 84.54 ± 0.68 | 100 | 100 |
| 8 | 58.96 ± 0.87 | 84.95 ± 0.26 | 100 | 100 |
| 9 | 48.21 ± 5.37 | 72.37 ± 1.12 | 95.94 ± 0.15 | 100 |
| 10 | 70.08 ± 0.10 | 94.70 ± 0.90 | 100 | 100 |
| 11 | 52.85 ± 0.05 | 79.07 ± 0.25 | 94.44 ± 0.37 | 98.67 ± 0.32 |
| 13 | 56.46 ± 0.79 | 82.78 ± 0.69 | 93.70 ± 0.44 | 98.9 ± 0.80 |
| 15 | 50.64 ± 1.64 | 80.67 ± 0.22 | 97.95 ± 0.45 | 94.99 ± 0.37 |
| 17 | 66.64 ± 1.30 | 86.17 ± 0.14 | 90.54 ± 0.24 | 95.4 ± 0.24 |
| 18 | 45.78 ± 1.84 | 73.44 ± 0.92 | 83.26 ± 0.23 | 86.46 ± 0.20 |
| 19 | 60.53 ± 2.02 | 89.44 ± 0.16 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferron, L.; Colombo, R.; Mannucci, B.; Papetti, A. A New Italian Purple Corn Variety (Moradyn) Byproduct Extract: Antiglycative and Hypoglycemic In Vitro Activities and Preliminary Bioaccessibility Studies. Molecules 2020, 25, 1958. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081958
Ferron L, Colombo R, Mannucci B, Papetti A. A New Italian Purple Corn Variety (Moradyn) Byproduct Extract: Antiglycative and Hypoglycemic In Vitro Activities and Preliminary Bioaccessibility Studies. Molecules. 2020; 25(8):1958. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081958
Chicago/Turabian StyleFerron, Lucia, Raffaella Colombo, Barbara Mannucci, and Adele Papetti. 2020. "A New Italian Purple Corn Variety (Moradyn) Byproduct Extract: Antiglycative and Hypoglycemic In Vitro Activities and Preliminary Bioaccessibility Studies" Molecules 25, no. 8: 1958. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081958