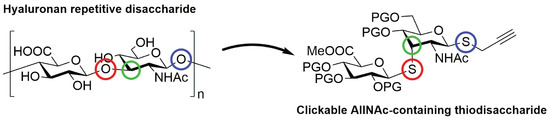
Synthetic Studies on the Incorporation of N-Acetylallosamine in Hyaluronic Acid-Inspired Thiodisaccharides
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Driguez, H. Thiooligosaccharides in glycobiology. Top. Curr. Chem. 1997, 187, 85–116. [Google Scholar] [CrossRef]
- Szilagyi, L.; Varela, O. Non-conventional Glycosidic Linkages: Syntheses and Structures of Thiooligosaccharides and Carbohydrates with Three-bond Glycosidic Connections. Curr. Org. Chem. 2006, 10, 1745–1770. [Google Scholar] [CrossRef]
- Sattin, S.; Bernardi, A. Design and synthesis of glycomimetics. Carbohydr. Chem. 2016, 41, 1–25. [Google Scholar] [CrossRef]
- Sarnik, J.; Gajek, A.; Toma, M.; Pawelczyk, J.; Rykowski, S.; Olejniczak, A.; Sliwinski, T.; Bielski, R.; Witczak, Z.J.; Poplawski, T. (1–4)-Thiodisaccharides as anticancer agents. Part 5. Evaluation of anticancer activity and investigation of mechanism of action. Bioorg. Med. Chem. Lett. 2020, 30, 126904. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Withers, S.G. Syntheses of the 3- and 4-thio analogues of 4-nitrophenyl 2-acetamido-2-deoxy-β-D-gluco- and galactopyranoside. Carbohydr. Res. 2007, 342, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Manzano, V.E.; Uhrig, M.L.; Varela, O. Straightforward synthesis of thiodisaccharides by ring-opening of sugar epoxides. J. Org. Chem. 2008, 73, 7224–7235. [Google Scholar] [CrossRef] [PubMed]
- Cagnoni, A.J.; Kovensky, J.; Uhrig, M.L. Design and synthesis of hydrolytically stable multivalent ligands bearing thiodigalactoside analogues for peanut lectin and human galectin-3 binding. J. Org. Chem. 2014, 79, 6456–6467. [Google Scholar] [CrossRef] [PubMed]
- Cristófalo, A.E.; Nieto, P.M.; Uhrig, M.L. Synthesis of (1→3) Thiodisaccharides of GlcNAc and the Serendipitous Formation of 2,3-Dideoxy-(1→2)-thiodisaccharides through a Vinyl Azide Intermediate. J. Org. Chem. 2020, 85, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Teki, D.S.E.K.; Bil, A.; Moreau, V.; Chagnault, V.; Fanté, B.; Adjou, A.; Kovensky, J. Synthesis of multivalent: S-glycoside analogs of a heparan sulfate sequence. Org. Chem. Front. 2019, 6, 2718–2725. [Google Scholar] [CrossRef]
- Cristófalo, A.E.; Cagnoni, A.J.; Uhrig, M.L. Synthesis of N-acetylglucosamine and N-acetylallosamine resorcinarene-based multivalent β-thio-glycoclusters: Unexpected affinity of N-acetylallosamine ligands towards Wheat Germ Agglutinin. Org. Biomol. Chem. 2020, 18, 6853–6865. [Google Scholar] [CrossRef]
- Griffith, D.A.; Danishefsky, S.J. Total Synthesis of Allosamidin: An Application of the Sulfonamidoglycosylation of Glycals. J. Am. Chem. Soc. 1991, 113, 5863–5864. [Google Scholar] [CrossRef]
- Maloisel, J.; Vasella, A.; Trost, B.M.; Vranken, D.L. Synthesis of Allosamidin. Helv. Chim. Acta 1992, 75, 1515–1526. [Google Scholar] [CrossRef]
- Andersen, O.A.; Dixon, M.J.; Eggleston, I.M.; van Aalten, D.M.F. Natural product family 18 chitinase inhibitors. Nat. Prod. Rep. 2005, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Inoue, H.; Nagasawa, H. Novel biological activities of allosamidins. Molecules 2013, 18, 6952–6968. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Kimura, M.; Miyazaki, H.; Okawa, K.; Onuki, R.; Nemoto, C.; Tabata, E.; Wakita, S.; Kashimura, A.; Sakaguchi, M.; et al. Acidic mammalian chitinase is a proteases-resistant glycosidase in mouse digestive system. Sci. Rep. 2016, 6, 37756. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Wang, P.; Huo, C.X.; Lang, S.; Caution, K.; Nick, S.T.; Dubey, P.; Deora, R.; Huang, X. Chemical Synthesis and Immunological Evaluation of a Pentasaccharide Bearing Multiple Rare Sugars as a Potential Anti-pertussis Vaccine. Angew. Chem. Int. Ed. 2020, 59, 6451–6458. [Google Scholar] [CrossRef]
- Křen, V.; Řezanka, T. Sweet antibiotics—The role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.A.; Pradhan, K.; Paul, A.; Olin, I.R.; Tuck, O.T.; Moulton, K.D.; Kulkarni, S.S.; Dube, D.H. Metabolic inhibitors of bacterial glycan biosynthesis. Chem. Sci. 2020, 11, 1761–1774. [Google Scholar] [CrossRef] [Green Version]
- Maloisel, J.; Vasella, A. Synthesis of N-Acetylallosamine-Derived Disaccharides. Helv. Chim. Acta 1992, 75, 1491–1514. [Google Scholar] [CrossRef]
- Strenge, K.; Schauer, R.; Bovin, N.; Hasegawa, A.; Ishida, H.; Kiso, M.; Kelm, S. Glycan specificity of myelin-associated glycoprotein and sialoadhesin deduced from interactions with synthetic oligosaccharides. Eur. J. Biochem. 1998, 258, 677–685. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A. Viscoeleastic properties of hyaluronan and its therapeutic use. In Chemistry and Biology of Hyaluronan; Garg, H.G., Hales, C.A., Eds.; Elsevier Press: Ámsterdam, The Netherlands, 2004; pp. 415–455. [Google Scholar]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hascall, V.C.; Esko, J.D. Hyaluronan. In Essentials of Glycobiology [Internet]; Varki, A., Cummings, R., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Lompardía, S.L.; Díaz, M.; Papademetrio, D.L.; Mascaró, M.; Pibuel, M.; Álvarez, E.; Hajos, S.E. Hyaluronan oligomers sensitize chronic myeloid leukemia cell lines to the effect of imatinib. Glycobiology 2016, 26, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristófalo, A.E.; Montenegro, H.O.; Cano, M.E.; Colomer, J.P.; Uhrig, M.L. Synthesis of 2-Propynyl 2-Acetamido-3,4,6-tri-O-Acetyl-2-Deoxy-1-Thio-β-D-Glucopyranoside, 2-Propynyl 3,4,6-tri-O-Acetyl-2-Deoxy-2-Phthalimido-1-Thio-β-D-Glucopyranoside and their 2-(2-Propynyloxyethoxy)ethyl Analogs. In Carbohydrate Chemistry: Proven Synthetic Methods Vol. 5; Kosma, P., Wrodnigg, T.M., Stütz, A., Eds.; CRC Press: Boca Raton, FL, USA, 2020; Volume 5, pp. 215–225. [Google Scholar]
- Cai, L.; Guan, W.; Kitaoka, M.; Shen, J.; Xia, C.; Chen, W.; Wang, P.G. A chemoenzymatic route to N-acetylglucosamine-1-phosphate analogues: Substrate specificity investigations of N-acetylhexosamine 1-kinase. Chem. Commun. 2009, 2944–2946. [Google Scholar] [CrossRef]
- Yerien, D.E.; Barata-Vallejo, S.; Camps, B.; Cristófalo, A.E.; Cano, M.E.; Uhrig, M.L.; Postigo, A. Electron-catalyzed radical perfluoroalkylation of organic sulfides: The serendipitous use of the TMEDA/I2 complex as a radical initiator. Catal. Sci. Technol. 2017, 7, 2274–2282. [Google Scholar] [CrossRef]
- Floyd, N.; Vijayakrishnan, B.; Koeppe, J.R.; Davis, B.G. Thiyl Glycosylation of Olefinic Proteins: S-Linked Glycoconjugate Synthesis. Angew. Chem. Int. Ed. Engl. 2009, 48, 7798–7802. [Google Scholar] [CrossRef]
- Nigudkar, S.S.; Demchenko, A.V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704. [Google Scholar] [CrossRef] [Green Version]
- Karban, J.; Kroutil, J. Chemistry of Carbohydrate Aziridines. Adv. Carbohydr. Chem. Biochem. 2006, 60, 27–101. [Google Scholar] [PubMed]
- Fowler, P.; Bernet, B.; Vasella, A. A 1H-NMR Spectroscopic Investigation of the Conformation of the Acetamido Group in Some Derivatives of N-Acetyl-D-allosamine and D-glucosamine. Helv. Chim. Acta 1996, 79, 269–287. [Google Scholar] [CrossRef]
- Crich, D.; Dudkin, V. Why are the hydroxy groups of partially protected N-acetylglucosamine derivatives such poor glycosyl acceptors, and what can be done about it? A comparative study of the reactivity of N-acetyl-, N-phthalimido-, and 2-azido-2-deoxy-glucosamine derivatives. J. Am. Chem. Soc. 2001, 123, 6819–6825. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-Y.; Kulkarni, S.S.; Chou, C.-H.; Liao, W.-M.; Hung, S.-C. A Concise Synthesis of Tetrahydroxy-LCB, α-Galactosyl Ceramide, and 1,4-Dideoxy-1,4-imino-L-ribitol via D-Allosamines as Key Building Blocks. J. Org. Chem. 2006, 71, 1226–1229. [Google Scholar] [CrossRef]
- Yeung, B.K.S.; Hill, D.C.; Janicka, M.; Petillo, P.A. Synthesis of two hyaluronan trisaccharides. Org. Lett. 2000, 2, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, R.V.; Shangguan, N.; Sauers, R.R.; Williams, L.J. Mechanism of thio acid/azide amidation. J. Am. Chem. Soc. 2006, 128, 5695–5702. [Google Scholar] [CrossRef] [PubMed]






 |  |  | |
|---|---|---|---|
| H-1 | 4.63 (J1,2 = 10.5) | 5.62 (J1,2 = 8.7) | 5.96 (J1,2 = 8.3) |
| H-2 | 4.52 (J2,3 = 3.9) | 3.94 (J2,3 = 4.7) | 3.81–3.71 2 |
| H-3 | 3.68 (J3,4 = 3.9) | 4.01 (J3,4 = 3.5) | 3.81–3.71 2 |
| H-4 | 3.89 (J4,5 = 8.5) | 3.87 (J4,5 = 9.2) | 3.81–3.71 2 |
| H-5 | 3.76 2 | 4.15 (J5,6eq = 5.2, J5,6ax = 10.3) | 3.81–3.71 2 |
| H-6ax | 3.76 2 | 3.74 (J6eq,6ax ≅ 10.3) | 3.68 (J6eq,6ax ≅ J5,6ax ≅ 10.2) |
| H-6eq | 4.32 2 | 4.34 | 4.36 (J5,6eq = 4,5) |
| H-1′ | 4.68 (J1′,2′ = 10.2) | 5.07 (J1′,2′ = 10.1) | 5.90 (J1′,2′ = 5.5) |
| H-2′ | 5.02 (J2′,3′ = 9.6) | 5.03 (J2′,3′ = 8.7) | 5.08 (J2′,3′ = 10.2) |
| H-3′ | 5.23 (J3′,4′ = 9.6) | 5.13 (J3′,4′ = 9.5) | 5.46 (J3′,4′ ≅ 10.2) |
| H-4′ | 5.16 (J4′,5′ = 9.6) | 5.22 (J4′,5′ = 9.8) | 5.09 (J4′,5′ ≅ 10.2) |
| H-5′ | 3.92 | 3.81 | 4.84 |
| C-1 | 82.9 | 92.1 | 92.7 |
| C-3 | 52.4 | 46.8 | 46.1 |
| C-1′ | 86.0 | 84.4 | 82.4 |
| C-5′ | 75.9 | 76.3 | 68.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristófalo, A.E.; Uhrig, M.L. Synthetic Studies on the Incorporation of N-Acetylallosamine in Hyaluronic Acid-Inspired Thiodisaccharides. Molecules 2021, 26, 180. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010180
Cristófalo AE, Uhrig ML. Synthetic Studies on the Incorporation of N-Acetylallosamine in Hyaluronic Acid-Inspired Thiodisaccharides. Molecules. 2021; 26(1):180. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010180
Chicago/Turabian StyleCristófalo, Alejandro E., and María Laura Uhrig. 2021. "Synthetic Studies on the Incorporation of N-Acetylallosamine in Hyaluronic Acid-Inspired Thiodisaccharides" Molecules 26, no. 1: 180. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010180