Sesquiterpenes from Myrrh and Their ICAM-1 Inhibitory Activity In Vitro
Abstract
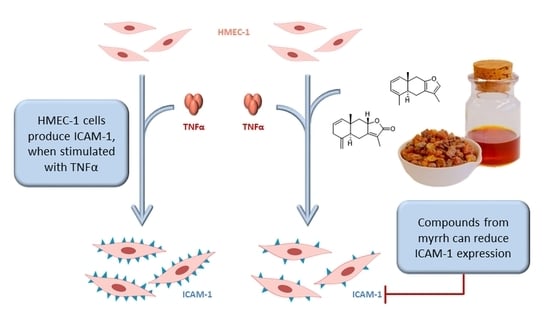
:1. Introduction
2. Results and Discussion
2.1. Isolated Compounds
2.2. Purity and ICAM-1 Inhibition
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction
3.3. Isolation
3.3.1. Liquid–liquid Partition
3.3.2. Silica Flash 1
3.3.3. CPC (Centrifugal Partition Chromatography)
3.3.4. Silica Flash 2
3.3.5. TLC (Thin Layer Chromatography)
3.3.6. Preparative HPLC (High-Performance Liquid Chromatography)
3.4. NMR
3.5. UHPLC-MS
3.6. Optical Methods
3.7. Purity
3.8. Isolates
3.8.1. 9-Nor-9,10-seco-isolindestrenolide (1)
3.8.2. 9,10-Seco-isohydroxylindestrenolide (2)
3.8.3. Myrrhanoperoxide (3)
3.8.4. rel-(+)-(1S,4R,7S)-11-Acetyl-guai-10(14)-en-4,11-ol (4)
3.8.5. rel-(+)-(4R,5R,7S)-11-Acetyl-guai-1(10)-en-4,11-ol (5)
3.8.6. Commiterpene D (6)
3.8.7. Lindestrenolide (7)
3.8.8. Isohydroxylindestrenolide (8)
3.8.9. Hydroxylindestrenolide (9)
3.8.10. Atractylenolide (10)
3.8.11. Commiphorane E3 (11)
3.8.12. 4β-Hydroxy-8,12-epoxyeudesma-7,11-diene-1,6-dione (12)
3.8.13. Isogermafurenolide (13)
3.8.14. Hydroxyisogermafurenolide (14)
3.8.15. 8-Epi-2-methoxyisogermafurenolide (15)
3.8.16. Methoxyisogermafurenolide (16)
3.9. Cell Culture
3.9.1. Cultivation
3.9.2. MTT Assay
3.9.3. ICAM-1 Assay
3.10. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shen, T.; Li, G.-H.; Wang, X.-N.; Lou, H.-X. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012, 142, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, W.; Hänsel, R.; Keller, K.; Reichling, J.; Rimpler, H.; Schneider, G. Hagers Handbuch der Pharmazeutischen Praxis, 5. Aufl.; Springer: Berlin, Germany, 2013; ISBN 9783642637261. [Google Scholar]
- Martinetz, D.; Lohs, K.; Janzen, J. Weihrauch und Myrrhe: Kulturgeschichte und Wirtschaftliche Bedeutung: Botanik, Chemie, Medizin; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1989; ISBN 3804710190. [Google Scholar]
- Tipton, D.A.; Lyle, B.; Babich, H.; Dabbous, M.K. In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol. In Vitro 2003, 17, 301–310. [Google Scholar] [CrossRef]
- Vissiennon, C.; Hammoud, D.; Rodewald, S.; Fester, K.; Goos, K.H.; Nieber, K.; Arnhold, J. Chamomile flower, myrrh, and coffee charcoal, components of a traditional herbal medicinal product, diminish proinflammatory activation in human macrophages. Planta Med. 2017, 83, 846–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-inflammatory and barrier-stabilizing effects of myrrh, coffee charcoal and chamomile flower extract in a co-culture cell model of the intestinal mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Bae, G.-S.; Park, K.-C.; Koo, B.S.; Kim, B.-J.; Lee, H.-J.; Seo, S.-W.; Shin, Y.K.; Jung, W.-S.; Cho, J.-H.; et al. Myrrh inhibits LPS-induced inflammatory response and protects from cecal ligation and puncture-induced sepsis. Evid.-Based Complement. Altern. Med. 2012, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatani, A.J.; Alrojayee, F.S.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Exp. Ther. Med. 2016, 12, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Langhorst, J.; Varnhagen, I.; Schneider, S.B.; Albrecht, U.; Rueffer, A.; Stange, R.; Michalsen, A.; Dobos, G.J. Randomised clinical trial: A herbal preparation of myrrh, chamomile and coffee charcoal compared with mesalazine in maintaining remission in ulcerative colitis - a double-blind, double-dummy study. Aliment. Pharmacol. Ther. 2013, 38, 490–500. [Google Scholar] [CrossRef]
- Lobatón, T.; Vermeire, S.; van Assche, G.; Rutgeerts, P. Review article: Anti-adhesion therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2014, 39, 579–594. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An update on inflammatory bowel disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C.; Banks, R.E.; Haidar, A.; Gearing, A.J.; Hemingway, I.K.; Ibbotson, S.H.; Dixon, M.F.; Axon, A.T. Adhesion molecules in inflammatory bowel disease. Gut 1995, 36, 724–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjunpää, H.; Llort, A.M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, E.; Hulett, M.; Ahrens, R.; Wagner, N.; Smart, V.; Matthaei, K.I.; Brandt, E.B.; Dent, L.A.; Rothenberg, M.E.; Tang, M.; et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J. Leukoc. Biol 2006, 80, 330–341. [Google Scholar] [CrossRef]
- Hogan, S.P. Functional role of eosinophils in gastrointestinal inflammation. Immunol. Allergy Clin. N. Am. 2009, 29, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Loktionov, A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J. Gastroenterol. 2019, 25, 3503–3526. [Google Scholar] [CrossRef]
- Hosten, T.A.; Zhao, K.; Han, H.Q.; Liu, G.; He, X.H. Alicaforsen: An emerging therapeutic agent for ulcerative colitis and refractory pouchitis. Gastroenterol. Res. 2014, 7, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Jakupovic, J.; Tan, R.X.; Bohlmann, F.; Jia, Z.J.; Huneck, S. Seco- and nor-sesquiterpene lactones with a new carbon skeleton from Artemisia santolinifolia. Phytochemystry 1991, 30, 19941–19946. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Baxter, R.L.; Chiang, C.-K.; Gilmore, C.J.; Bryan, R.F. Eriolangin and eriolanin, novel antileukaemic seco-eudesmanolides from Eriophyllum lanatum. J. Chem. Soc. Chem. Commun. 1973, 842–843. [Google Scholar] [CrossRef]
- Xu, J.J.; Tan, N.H.; Xiong, J.; Adebayo, A.H.; Han, H.J.; Zeng, G.Z.; Ji, C.J.; Zhang, Y.M.; Zhu, M.J. Oxyphyllones A and B, novel sesquiterpenes with an unusual 4,5-secoeudesmane skeleton from Alpinia oxyphylla. Chin. Chem. Lett. 2009, 20, 945–948. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, H.-L.; Zhang, J.; Du, T.; Guo, E.-Y.; Liu, W.-Y.; Luo, J.-G.; Ye, F.; Feng, F.; Qu, W. Sesquiterpenoids from Chloranthus anhuiensis with neuroprotective effects in PC12 cells. J. Nat. Prod. 2018, 81, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Gáti, T.; Hussein, T.A.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Tóth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1,10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Zare, K.; Biniyaz, T.; Fazlalizadeh, G. A seco-guaianolide and other sesquiterpene lactones from Postia bombycina. Phytochemystry 1989, 28, 3127–3129. [Google Scholar] [CrossRef]
- Pereira, M.D.P.; da Silva, T.; Lopes, L.M.X.; Krettli, A.U.; Madureira, L.S.; Zukerman-Schpector, J. 4,5-seco-guaiane and a nine-membered sesquiterpene lactone from Holostylis reniformis. Molecules 2012, 17, 14046–14057. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Zdero, C. Sesquiterpene lactones and other constituents from Tanacetum parthenium. Phytochemistry 1982, 21, 2543–2549. [Google Scholar] [CrossRef]
- Liu, L.; Dai, W.; Xiang, C.; Chi, J.; Zhang, M. 1,10-Secoguaianolides from Artemisia austro-yunnanensis and their anti-inflammatory effects. Molecules 2018, 23, 1639. [Google Scholar] [CrossRef]
- Huneck, S.; Zdero, C.; Bohlmann, F. Seco-guaianolides and other constituents from Artemisia species. Phytochemistry 1986, 25, 883–889. [Google Scholar] [CrossRef]
- Zhou, Q.-M.; Chen, M.-H.; Li, X.-H.; Peng, C.; Lin, D.-S.; Li, X.-N.; He, Y.; Xiong, L. Absolute configurations and bioactivities of guaiane-type sesquiterpenoids isolated from Pogostemon cablin. J. Nat. Prod. 2018, 81, 1919–1927. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Ma, X.-Y.; Cai, X.-Q.; Yan, P.-C.; Yue, L.; Lin, C.; Shao, W.-W. Sesquiterpenoids from Curcuma wenyujin with anti-influenza viral activities. Phytochemistry 2013, 85, 122–128. [Google Scholar] [CrossRef]
- de Pascual-T, J.; Bellido, I.S.; González, M.S. Chenopodiaceae components: Polyoxigenated sesquiterpenes from Chenopodium botrys. Tetrahedron 1980, 36, 371–376. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Zhao, P.; Xie, C.; Jin, D.-Q.; Hou, W.; Zhang, T. Neuroprotective cadinane sesquiterpenes from the resinous exudates of Commiphora myrrha. Fitoterapia 2011, 82, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; He, S.; Wu, X.-D.; Wu, D.-K.; Pan, Y.-J. Cadinane and eudesmane sesquiterpenoids from Chloranthus henryi. Helv. Chim. Acta 2007, 90, 1586–1592. [Google Scholar] [CrossRef]
- Takeda, K.; Horibe, I.; Minato, H. Components of the root of Lindera strychnifolia Vill. Part XIV. Sesquiterpene lactones from the root of Lindera strychnifolia Vill. J. Chem. Soc. 1968, 569–572. [Google Scholar] [CrossRef]
- Greve, H.L.; Kaiser, M.; Schmidt, T.J. Investigation of antiplasmodial effects of terpenoid compounds isolated from myrrh. Planta Med. 2020, 86, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wan, W.-Z.; Wang, X.-N.; Yuan, H.-Q.; Ji, M.; Lou, H.-X. A triterpenoid and sesquiterpenoids from the resinous exudates of Commiphora myrrha. Helv. Chim. Acta 2009, 92, 645–652. [Google Scholar] [CrossRef]
- Endo, K.; Taguchi, T.; Taguchi, F.; Hikino, H.; Yamahara, J.; Fujimura, H. Antiinflammatory principles of Atractylodes rhizomes. Chem. Pharm. Bull. 1979, 27, 2954–2958. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Luo, Q.; Cheng, L.-Z.; Yan, Y.-M.; Cheng, Y.-X.; Wang, S.-M. New terpenoids from Resina Commiphora. Fitoterapia 2017, 117, 147–153. [Google Scholar] [CrossRef]
- Wu, B.; He, S.; Wu, X.-D.; Pan, Y.-J. New tyrosinase inhibitory sesquiterpenes from Chloranthus henryi. Chem. Biodivers. 2008, 5, 1298–1303. [Google Scholar] [CrossRef]
- Freischmidt, A.; Jürgenliemk, G.; Kraus, B.; Okpanyi, S.N.; Müller, J.; Kelber, O.; Weiser, D.; Heilmann, J. Contribution of flavonoids and catechol to the reduction of ICAM-1 expression in endothelial cells by a standardised willow bark extract. Phytomedicine 2012, 19, 245–252. [Google Scholar] [CrossRef]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. Biol. Chem. 1997, 378, 951–961. [Google Scholar] [CrossRef]
- Garcıía-Piñeres, A.J.; Lindenmeyer, M.T.; Merfort, I. Role of cysteine residues of p65/NF-κB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004, 75, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Chen, Y.; Shinozaki, M.; Arao, K.; Wang, L.; Tang, W.; Hirano, S.; Ogura, H.; Mitsui, T.; Taketani, S.; et al. Eudesmane-type sesquiterpene lactones inhibit multiple steps in the NF-κB signaling pathway induced by inflammatory cytokines. Bioorg. Med. Chem. Lett. 2012, 22, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C.; Escandell, J.M.; Andújar, I. Inhibition of transcription factors by plant-derived compounds and their implications in inflammation and cancer. Curr. Pharm. Des. 2009, 15, 1212–1237. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem 2001, 276, 39713–39720. [Google Scholar] [CrossRef] [Green Version]
- Amslinger, S. The tunable functionality of alpha, beta-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem 2010, 5, 351–356. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-activity relationships of sesquiterpene lactones. Stud. Nut. Prod. Chem. 2006, 33, 309–392. [Google Scholar] [CrossRef]
- Rosenthal, R.; Luettig, J.; Hering, N.A.; Krug, S.M.; Albrecht, U.; Fromm, M.; Schulzke, J.-D. Myrrh exerts barrier-stabilizing and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells. Int. J. Colorectal. Dis. 2017, 32, 623–634. [Google Scholar] [CrossRef]
- Marston, A.; Borel, C.; Hostettmann, K. Separation of natural products by centrifugal partition chromatography. J. Chromatogr. A 1988, 450, 91–99. [Google Scholar] [CrossRef]









| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 7.02 (1H, d, 7.4) | 128.1 | 7.04 (1H, d, 7.7) | 128.3 | 2.60 (1H, ddd, 6.2, 6.2, 6.2) | 52.3 |
| 2 | 7.08 (1H, dd, 7.4, 7.4) | 126.7 | 7.11 (1H, dd, 7.7, 7.7) | 127.3 | 1.69 (1H, dddd, 6.6, 6.6, 6.6, 13.0) 2.05 (1H, dddd, 6.5, 6.5, 6.5, 13.0) | 30.4 |
| 3 | 7.02 (1H, d, 7.4) | 128.1 | 7.04 (1H, d, 7.7) | 128.3 | 1.79 (1H, ddd, 6.9, 6.9, 13.5) 1.92 (1H, ddd, 6.9, 6.9, 13.5) | 37.0 |
| 4 | 134.1 | 137.1 | 95.2 | |||
| 5 | 137.0 | 132.5 | 3.19 (1H, dd, 5.5, 5.5) | 66.4 | ||
| 6 | 3.66 (2H, s) | 25.7 | 3.69 (1H, d, 18.7) 3.80 (1H, d, 18.7) | 27.4 | 4.79 1 (1H, m) | 83.4 |
| 7 | 156.2 | 157.8 | 161.1 | |||
| 8 | 4.55 (2H, s) | 72.8 | 105.3 | 4.64 (2H, s) | 72.7 | |
| 9 | 1.58 (3H, s) | 24.3 | 4.76 (1H, brs) 4.78 1 (1H, s) | 110.1 | ||
| 10 | 134.0 | 137.1 | 146.0 | |||
| 11 | 123.9 | 124.7 | 123.0 | |||
| 12 | 175.0 | 171.7 | 172.8 | |||
| 13 | 1.43 (3H, s) | 11.1 | 1.29 (3H, brs) | 7.5 | 2.22 (3H, s) | 12.9 |
| 14 | 2.27 (3H, s) | 20.3 | 2.28 (3H, s) | 20.1 | 1.76 (3H, s) | 21.5 |
| 15 | 2.27 (3H, s) | 20.3 | 2.28 (3H, s) | 20.1 | 1.46 (3H, s) | 24.2 |
| No. | 4 | 5 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 2.23 (1H, ddd, 8.1, 17.5) | 47.8 | 135.4 | |
| 2 | 1.68 1 (1H, m) 1.86 1 (1H, m) | 25.6 | 2.19 1 (1H, m) 2.30 1 (1H, m) | 28.4 |
| 3 | 1.72 1 (2H, m) | 40.8 | 1.68 1 (2H, m) | 39.2 |
| 4 | 81.2 | 81.0 | ||
| 5 | 1.73 1 (1H, m) | 52.7 | 2.65 (1H, brd, 11,2) | 49.3 |
| 6 | 1.51 (1H, ddd, 7.7, 11.4, 14.1) 1.64(1H, ddd, 5.3, 9.1, 14.2) | 27.2 | 1.51 (1H, ddd, 3.6, 7.8, 14.4) 1.72 1 (1H, m) | 27.9 |
| 7 | 2.42 (1H, dddd, 5.3, 7.9, 7.9, 10.9) | 44.2 | 2.26 1 (1H, m) | 43.4 |
| 8 | 1.29 (1H, dddd, 2.7, 11.1, 11.1, 13.9) 1.75 1 (1H, m) | 26.9 | 1.65 1 (2H, m) | 24.9 |
| 9 | 2.05 (1H, m) 2.53 (1H, ddd, 2.6, 6.6, 14.1) | 38.2 | 2.11 1 (1H, m) 2.25 1 (1H, m) | 34.8 |
| 10 | 153.1 | 127.7 | ||
| 11 | 86.2 | 86.3 | ||
| 12 | 1.41 (3H, s) | 23.2 | 1.44 (3H, s) | 23.4 |
| 13 | 1.41 (3H, s) | 23.4 | 1.44 (3H, s) | 23.7 |
| 14 | 4.68 (1H, s) 4.70 (1H, s) | 106.3 | 1.55 (3H, s) | 20.9 |
| 15 | 1.20 (3H, s) | 23.8 | 1.11 (3H, s) | 22.2 |
| 1′-OAc | 170.5 | 170.5 | ||
| 2′-OAc | 1.97 (3H, s) | 22.6 | 1.97 (3H, s) | 22.7 |
| No. | 6 | 7 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 131.7 | 5.55 1 (1H, m) | 136.5 | |
| 2 | 4.30 (1H, dd, 2.9, 2.9) | 73.1 | 5.55 1 (1H, m) | 123.6 |
| 3 | 1.60 1 (1H, m) 2.21 (1H, ddd, 2.9, 2.9, 14.4) | 31.6 | 2.79 (1H, dd, 19.2) 2.92 (1H, dd, 19.0) | 34.7 |
| 4 | 2.30 (1H, m) | 30.3 | 145.4 | |
| 5 | 5.87 (1H, d, 9.4) | 74.9 | 2.18 (1H, dd, 3.3, 13.1) | 48.1 |
| 6 | 133.0 | 2.52 (1H, dd, 13.1, 14.0) 2.85 (1H, dd, 3.5, 14.0) | 24.6 | |
| 7 | 124.0 | 164.1 | ||
| 8 | 153.4 | 4.97 1 (1H, m) | 78.8 | |
| 9 | 6.96 (1H, s) | 112.9 | 1.20 (1H, dd, 11.9) 2.37 (1H, dd, 6.6, 11.9) | 44.6 |
| 10 | 140.3 | 37.8 | ||
| 11 | 3.61 (1H, q, 7.4) | 38.9 | 119.6 | |
| 12 | 178.3 | 175.8 | ||
| 13 | 1.47 (3H, d, 7.4) | 15.5 | 1.80 (3H, s) | 6.7 |
| 14 | 2.39 (3H, s) | 19.5 | 0.98 (3H, s) | 18.2 |
| 15 | 1.09 (3H, d, 6.6) | 19.1 | 4.81 (1H, s) 4.99 (1H, s) | 106.5 |
| 1′-OAc | 171.1 | |||
| 2′-OAc | 2.17 (3H, s) | 20.9 | ||
| 1″-Me | 3.45 (3H, s) | 56.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuck, K.; Jürgenliemk, G.; Lipowicz, B.; Heilmann, J. Sesquiterpenes from Myrrh and Their ICAM-1 Inhibitory Activity In Vitro. Molecules 2021, 26, 42. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010042
Kuck K, Jürgenliemk G, Lipowicz B, Heilmann J. Sesquiterpenes from Myrrh and Their ICAM-1 Inhibitory Activity In Vitro. Molecules. 2021; 26(1):42. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010042
Chicago/Turabian StyleKuck, Katrin, Guido Jürgenliemk, Bartosz Lipowicz, and Jörg Heilmann. 2021. "Sesquiterpenes from Myrrh and Their ICAM-1 Inhibitory Activity In Vitro" Molecules 26, no. 1: 42. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010042