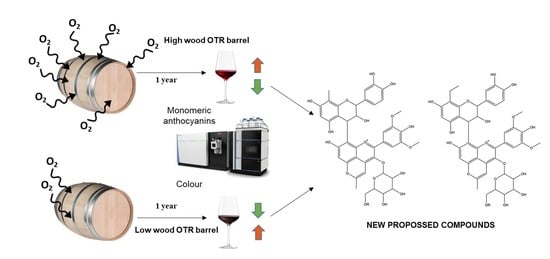
Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels
Abstract
:1. Introduction
2. Results
2.1. Oenological Parameters and Color
2.2. Anthocyanin Content
2.3. Anthocyanin Identification by LC-MS
3. Materials and Methods
3.1. Oak Wood Barrels
3.2. Red Wine and Color Parameters
3.3. Anthocyanin Quantification
3.4. Anthocyanin Derivatives Identification
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freitas, V.; Mateus, N. Chemical transformations of anthocyanins yielding a variety of colours (Review). Environ. Chem. Lett. 2006, 4, 175–183. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; Freitas, V. Wine-inspired chemistry: Anthocyanin transformations for a portfolio of natural colors. Synlett 2017, 28, 898–906. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; Freitas, V. Previous and recent advances in pyranoanthocyanins equilibria in aqueous solution. Dye. Pigment. 2014, 100, 190–200. [Google Scholar] [CrossRef]
- Cheynier, V.; Salas, E.; Souquet, J.; Sarni-manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 3, 298–305. [Google Scholar]
- Fernandes, A.; Oliveira, J.; Mateus, N.; Freitas, V. A review of the current knowledge of red wine colour. J. Int. des Sci. la Vigne du Vin 2016, 51, 1–21. [Google Scholar]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Lee, D.F.; Swinny, E.E.; Asenstorfer, R.E.; Jones, G.P. Factors affecting the formation of red wine pigments. ACS Symp. Ser. 2004, 886, 125–142. [Google Scholar]
- Cano-López, M.; Pardo-Minguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Chromatic characteristics and anthocyanin profile of a micro-oxygenated red wine after oak or bottle maturation. Eur. Food Res. Technol. 2007, 225, 127–132. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Alamo-Sanza, M.; Nevares, I. Recent advances in the evaluation of the oxygen transfer rate in oak barrels. J. Agric. Food Chem. 2014, 62, 8892–8899. [Google Scholar] [CrossRef] [PubMed]
- Alamo-Sanza, M.; Cárcel, L.M.; Nevares, I. Characterization of the Oxygen Transmission Rate of Oak Wood Species Used in Cooperage. J. Agric. Food Chem. 2017, 65, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álamo-Sanza, M.; Nevares Domínguez, I. Oxygen transfer rate in oak barrels. Annual evaluation for dynamic oxygen intake and entry. Wines Vines 2015, 12, 58–64. [Google Scholar]
- Martínez-Martínez, V.; Alamo-Sanza, M.; Nevares, I. Application of image analysis and artificial neural networks to the prediction in-line of OTR in oak wood planks for cooperage. Mater. Des. 2019, 181, 107979. [Google Scholar] [CrossRef]
- Prat-García, S.; Nevares, I.; Martínez-Martínez, V.; Alamo-Sanza, M. Customized oxygenation barrels as a new strategy for controlled wine aging. Food Res. Int. 2020, 131, 108982. [Google Scholar] [CrossRef]
- Martínez-Martínez, V.; Nevares, I.; Alamo-Sanza, M. Artificial Intelligence Methods for Constructing Wine Barrels with a Controlled Oxygen Transmission Rate. Molecules 2020, 25, 3312. [Google Scholar] [CrossRef]
- Nevares, I.; Alamo, M.; Cárcel, L.M.; Crespo, R.; Martin, C.; Gallego, L. Measure the dissolved oxygen consumed by red wines in aging tanks. Food Bioprocess Technol. 2009, 2, 328–336. [Google Scholar] [CrossRef]
- Vidal, S.; Meudec, E.; Cheynier, V.; Skouroumounis, G.; Hayasaka, Y. Mass Spectrometric Evidence for the Existence of Oligomeric Anthocyanins in Grape Skins. J. Agric. Food Chem. 2004, 52, 7144–7151. [Google Scholar] [CrossRef]
- Álamo Sanza, M.; Nevares Domínguez, I.; García Merino, S. Influence of different aging systems and oak woods on aged wine color and anthocyanin composition. Eur. Food Res. Technol. 2004, 219, 124–132. [Google Scholar] [CrossRef]
- Mateus, N.; De Freitas, V. Evolution and Stability of Anthocyanin-Derived Pigments during Port Wine Aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.K.; Shi, Y.; Duan, C.Q.; He, F. Reaction kinetics of the acetaldehyde-mediated condensation between (−)-epicatechin and anthocyanins and their effects on the color in model wine solutions. Food Chem. 2019, 283, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. What Gives a Wine Its Strong Red Color? Main Correlations Affecting Copigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Revilla, E.; López, J.F.; Ryan, J.M. Anthocyanin pattern of Tempranillo wines during ageing in oak barrels and storage in stainless-steel tanks. Eur. Food Res. Technol. 2005, 220, 592–596. [Google Scholar] [CrossRef]
- Oliveira, J.; Alhinho Da Silva, M.; Teixeira, N.; Freitas, V.; Salas, E. Screening of Anthocyanins and Anthocyanin-Derived Pigments in Red Wine Grape Pomace Using LC-DAD/MS and MALDI-TOF Techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The Chemistry of Wine PolyphenolicC-Glycosidic Ellagitannins Targeting Human Topoisomerase II. Chem. A Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Prat-García, S.; Martínez-Martínez, V.; Alamo-Sanza, M.; Müller, B.J.; Mayr, T.; Nevares, I. Image of O2 dynamics released by oak wood submerged in model wine with nanoparticle sensors. Sens. Actuators B Chem. 2019, 284, 337–345. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Quaglieri, C.; Jourdes, M.; Waffo-Teguo, P.; Teissedre, P.L. Updated knowledge about pyranoanthocyanins: Impact of oxygen on their contents, and contribution in the winemaking process to overall wine color. Trends Food Sci. Technol. 2017, 67, 139–149. [Google Scholar] [CrossRef]
- Mateus, N.; Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; Freitas, V. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nave, F.; Teixeira, N.; Mateus, N.; De Freitas, V. Hemisynthesis and structural characterization of flavanol-(4,8)-vitisins by mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Compendium of international analysis of methods—OIV Chromatic Characteristics. Method OIV-MA-AS2-11. Determination of chromatic characteristics according to CIELab. 2006. Available online: http://www.oiv.int/public/medias/2478/oiv-ma-as2-11.pdf (accessed on 31 October 2019).
- Glories, Y. La couleur des vins rouges 2. Mesure, origine et interprétation. Connaiss. Vigne Vin 1984, 18, 253–271. [Google Scholar] [CrossRef]


| Control Barrels | LW-OTR Barrels | HW-OTR Barrels | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± σ | C.V. | Mean ± σ | C.V. | Mean ± σ | C.V. | p-Value 1 | p-Value 2 | ||
| Alcoholic degree (% vol) | 6 months | 15.11 ± 0.01 | 0.094% | 15.08 ± 0.01 | 0.099% | 15.08 ± 0.02 | 0.14% | 0.1586 | 0.8555 |
| Total acidity (g/L) | 5.01 ± 0.02 | 0.42% | 4.95 ± 0.02 | 0.35% | 4.95 ± 0.04 | 0.73% | 0.0983 * | 0.8100 | |
| Volatil acidity (g/L) | 0.67 ± 0.02 | 3.19% | 0.70 ± 0.03 | 3.92% | 0.72 ± 0.01 | 1.81% | 0.0816 * | 0.4425 | |
| pH | 3.86 ± 0.01 | 0.18% | 3.85 ± 0.01 | 0.21% | 3.85 ± 0.00 | 0.13% | 0.6991 | 0.6202 | |
| Acetaldehyde (mg/L) | 8.50 ± 0.71 | 8.32% | 10.00 ± 0.82 | 8.16% | 10.00 ± 0.82 | 8.16% | 0.1278 | 1.00 | |
| Ethyl acetate (mg/L) | 71.50 ± 2.12 | 2.97% | 70.00 ± 1.83 | 2.61% | 64.50 ± 0.58 | 0.89% | 0.0012 *** | 0.001 *** | |
| CI | 18.45 ± 0.07 | 0.39% | 18.20 ± 0.40 | 2.19% | 17.94 ± 0.10 | 0.58% | 0.0177 ** | 0.0991 * | |
| %420 | 37.38 ± 0.10 | 0.26% | 37.21 ± 0.08 | 0.22% | 37.42 ± 0.05 | 0.13% | 0.0001 **** | 0.0000 **** | |
| %520 | 48.76 ± 0.15 | 0.31% | 48.91 ± 0.10 | 0.21% | 48.76 ± 0.06 | 0.12% | 0.0121 ** | 0.0024 *** | |
| %620 | 13.86 ± 0.07 | 0.51% | 13.88 ± 0.11 | 0.81% | 13.82 ± 0.02 | 0.17% | 0.3344 | 0.1659 | |
| Alcoholic degree (% vol) | 12 months | 15.31 ± 0.02 | 0.14% | 15.25 ± 0.01 | 0.093% | 15.26 ± 0.01 | 0.085% | 0.0087 *** | 0.6202 |
| Total acidity (g/L) | 4.66 ± 0.07 | 1.52% | 4.66 ± 0.03 | 0.62% | 4.72 ± 0.02 | 0.46% | 0.0840 * | 0.0132 ** | |
| Volatil acidity (g/L) | 0.78 ± 0.04 | 5.44% | 0.76 ± 0.02 | 1.98% | 0.76 ± 0.02 | 2.40% | 0.5109 | 0.8394 | |
| pH | 3.84 ± 0.01 | 0.37% | 3.84 ± 0.01 | 0.22% | 3.83 ± 0.00 | 0% | 0.1930 | 0.0498 ** | |
| Acetaldehyde (mg/L) | 34.00 ± 2.83 | 8.31% | 34.50 ± 7.37 | 21.37% | 36.25 ± 9.74 | 26.88% | 0.9328 | 0.7841 | |
| Ethyl acetate (mg/L) | 60.50 ± 0.71 | 1.17% | 60.00 ± 2.58 | 4.30% | 62.00 ± 1.41 | 2.28% | 0.3815 | 0.2231 | |
| CI | 16.60 ± 0.34 | 2.07% | 16.84 ± 0.53 | 3.15% | 16.40 ± 0.25 | 1.51% | 0.1250 | 0.0535 * | |
| %420 | 38.92 ± 0.17 | 0.43% | 38.61 ± 0.32 | 0.82% | 38.79 ± 0.08 | 0.20% | 0.0852 * | 0.1545 | |
| %520 | 47.53 ± 0.09 | 0.20% | 47.71 ± 0.21 | 0.43% | 47.59 ± 0.04 | 0.09% | 0.1004 | 0.1134 | |
| %620 | 13.54 ± 0.08 | 0.56% | 13.68 ± 0.12 | 0.90% | 13.63 ± 0.07 | 0.48% | 0.1026 | 0.3443 | |
| Control Barrels | LW-OTR Barrels | HW-OTR Barrels | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± σ | C.V. | Mean ± σ | C.V. | Mean ± σ | C.V. | p-Value 1 | p-Value 2 | ||
| Dp-3-O-gluc | 6 months | 31.6 ± 1.3 | 4.2% | 28.9 ± 6.7 | 23.1% | 32.0 ± 1.0 | 3.3% | 0.3464 | 0.213 |
| Pt-3-O-gluc | 32.5 ± 1.3 | 4.0% | 31.3 ± 2.3 | 7.3% | 31.7 ± 1.5 | 4.8% | 0.5875 | 0.7057 | |
| Pn-3-O-gluc | 10.1 ± 0.5 | 4.6% | 10.5 ± 2.2 | 20.5% | 9.9 ± 0.4 | 4.1% | 0.6685 | 0.4210 | |
| Mv-3-O-gluc | 231.9 ± 5.5 | 2.4% | 215.7 ± 34.9 | 16.2% | 230.4 ± 4.7 | 2.0% | 0.3614 | 0.2573 | |
| Dp-3-O-acetylgluc | 2.1 ±0.1 | 3.1% | 2.1 ± 0.5 | 25.8% | 1.8 ±0.1 | 5.7% | 0.4542 | 0.3346 | |
| Pn 3-O-acetylgluc | 7.3 ± 0.4 | 6.0% | 6.5 ± 1.5 | 23.1% | 7.1 ± 0.2 | 3.2% | 0.3639 | 0.3676 | |
| Mv-3-O-gluc py | nq | nq | nq | ||||||
| Pt 3-O-acetylgluc | nd | nd | nd | ||||||
| Mv-3-O-acetylgluc | 74.2 ± 2.2 | 3.0% | 67.8 ± 12.2 | 17.9% | 72.0 ± 2.3 | 3.2% | 0.3838 | 0.3519 | |
| Mv-3-(6-p-coumaroyl)-gluc | 15.8 ± 0.6 | 4.0% | 15.4 ± 0.7 | 4.5% | 15.5 ±0.7 | 4.4% | 0.5622 | 0.8947 | |
| Total | 410.0 ± 12.0 | 378.2 ± 61 | 400.4 ± 10.9 | ||||||
| Dp-3-O-gluc | 13.4 ± 1.2 | 8.9% | 13.3 ± 1.0 | 7.5% | 14.4 ± 0.7 | 5.1% | 0.0657 * | 0.0241 ** | |
| Pt-3-O-gluc | 16.8 ± 1.0 | 6.2% | 16.6 ± 1.4 | 8.2% | 17.8 ± 0.4 | 2.3% | 0.068 * | 0.0278 ** | |
| Pn-3-O-gluc | 12 months | 8.3 ±0.4 | 5.1% | 7.9 ± 0.6 | 7.4% | 8.3 ± 0.2 | 2.6% | 0.1435 | 0.0651 |
| Mv-3-O-gluc | 127.1 ± 7.9 | 6.2% | 120.9 ± 12.0 | 9.9% | 130.3 ± 3.2 | 2.4% | 0.1193 | 0.0503 | |
| Dp-3-O-acetylgluc | nq | 5.6 ± 0.4 | 7.9% | 5.7 ± 0.2 | 4.3% | 0.8440 | 0.844 | ||
| Mv-3-O-gluc py | 7.1 ± 1.7 | 23.7% | 5.4 ± 0.2 | 3.2% | 5.8 ± 1.1 | 19.1% | 0.0351 ** | 0.3027 | |
| Pt 3-O-acetylgluc | 6.0 ± 0.4 | 6.0% | 5.7 ± 0.4 | 6.7% | 6.0 ± 0.8 | 2.9% | 0.1355 | 0.0513 * | |
| Pn 3-O-acetylgluc | 5.2 ± 0.0 | 0.2% | 4.7 ± 0.4 | 7.6% | 5.2 ± 0.1 | 1.8% | 0.0083 *** | 0.0058 *** | |
| Mv-3-O-acetylgluc | 45.5 ± 2.7 | 6.0% | 41.7 ± 4.6 | 11.0% | 46.0 ± 1.3 | 2.9% | 0.0438 ** | 0.0249 ** | |
| Mv-3-(6-p-coumaroyl)-gluc | 10.3 ± 0.8 | 7.9% | 9.7 ±1.0 | 10.4% | 10.0 ± 0.5 | 5.5% | 0.4914 | 0.5684 | |
| Total | 239.7 ± 16.1 | 231.5 ± 22.4 | 249.5 ± 8.5 | ||||||
| Compound | Rt (min) | [M+] | [M2] | [M3] | Identity | 6 Months | 12 Months |
|---|---|---|---|---|---|---|---|
| Anthocyanin-flavanol pigments | |||||||
| 1 | 16.76 | 797 | 635 | 467/373 | mv-3-O-gluc-gallocatechin | C, L, H | C, L, H |
| 2 | 27.26 | 781 | 619 | 467/373 | mv-3-O-gluc-cat [5] | C, L, H | C, L, H |
| 3 | 45.04 | 823 | 619 | 467/373 | mv-3-O-acetylgluc-(+)-catechin [5] | - | C, L, H |
| 4 | 48.93 | 823 | 619 | 467/373 | mv-3-O-acetylgluc-(−)-(epi)catechin | - | C, L, H |
| 5 | 55.67 | 927 | 619 | 467/373 | mv-3-O-coumaroylgluc-catechin | C, L, H | - |
| Monoglucoside anthocyanins | |||||||
| 6 | 28.53 | 465 | 303 | dp-3-O-gluc | C, L, H | C, L, H | |
| 7 | 31.46 | 449 | 287 | cy-3-O-gluc | C, L, H | - | |
| 8 | 36.02 | 479 | 317 | pt-3-O-gluc | C, L, H | C, L, H | |
| 9 | 40.5 | 463 | 301 | pn-3-O-gluc | C, L, H | C, L, H | |
| 10 | 41.74 | 493 | 331 | mv-3-O-gluc | C, L, H | C, L, H | |
| 11 | 46.34 | 507 | 303 | dp-3-O-acetylgluc | C, L, H | C, L, H | |
| 12 | 47.05 | 511 | 349 | mv-3-O-gluc chalcone form [4] | C, L, H | C, L, H | |
| 13 | 50.82 | 491 | 287 | cy-3-O-acetylgluc | C, L, H | - | |
| 14 | 51.34 | 565 | 331 | mv-3-O-lactgluc [10] | C, L, H | - | |
| 15 | 53.26 | 521 | 317 | pt-3-O-acetylgluc | C, L, H | C, L, H | |
| 16 | 53.94 | 553 | 349 | mv-3-O-acetgluc chalcone form [5] | C, L, H | C, L, H | |
| 17 | 59.65 | 535 | 331 | mv-3-O-acetylgluc | C, L, H | C, L, H | |
| 18 | 61.12 | 655 | 331 | mv-3-O-cafgluc [5] | C, H | - | |
| 19 | 62.5 | 611 | 303 | dp-3-O-coumgluc | C, L, H | C, L, H | |
| 20 | 68.2 | 625 | 317 | pt-3-O-coumgluc | C | C, H | |
| 21 | 68.57 | 639 | 331 | mv-3-O-coumgluc [5] | C, L, H | C, L, H | |
| Diglucoside anthocyanins | |||||||
| 22 | 28.78 | 641 | 317 | pt-3,5-O-digluc [10] | C,H | - | |
| 23 | 44.81 | 627 | 465 | 303 | dp-3,5-O-digluc [5] | C,H,L | C |
| Oligomeric anthocyanins | |||||||
| 24 | 32.3 | 1477 | 1315 | 1153 | mv-3-O-gluc trimer [18] | - | C, L, H |
| 25 | 49.2 | 1027 | 865 | 661 | mv-3-O-gluc-mv-O-acetgluc dimer [18] | - | C |
| 26 | 52.49 | 1029 | 849/ 357 | 687/342/ 295 | mv-3-gluc-ethyl-mv-3-gluc hydrated form [5] | - | C, L, H |
| 27 | 69.12 | 1131 | 823 | 661 | mv-3-O-coumgluc-mv-3-O-gluc dimer [10] | - | C, L, H |
| A-type vitisins | |||||||
| 28 | 47.3 | 561 | 399 | A-type vitisin [5] | C, L, H | C, L, H | |
| 29 | 51.68 | 603 | 399 | carboxypyranomv-3-O-acetylgluc | C, L, H | C, L, H | |
| 30 | 64.58 | 707 | 399 | carboxypyranomv-3-O-coumaroylgluc [20] | H | C, L, H | |
| B-type vitisins | |||||||
| 31 | 48.21 | 517 | 355 | B-type vitisin [22,23] | C, L, H | C, L, H | |
| 32 | 53.74 | 559 | 355 | pyranomv-3-O-acet-gluc | - | C, L, H | |
| Flavanol-methylpyranoanthocyanins | |||||||
| 33 | 48.6 | 819 | 657 | 531 | (+)-cat-methylpyranomv-3-O-gluc [5] | - | C, L, H |
| 34 | 57.77 | 819 | 657 | 531 | (−)-epicat-methylpyranomv-3-O-gluc | - | C, L, H |
| 35 | 67.26 | 861 | 657 | 531 | cat-methylpyranomv-3-O-acetylgluc | C, H | C, L, H |
| 36 | 75.34 | 965 | 657 | 531 | cat-methylpyranomv-3-O-coumgluc | C | - |
| Methyl-flavanol-methylpyranoanthocyanins | |||||||
| 37 | 66.1 | 833 | 671 | 531 | methyl-cat-methylpyranomv-3-gluc [5] | C, L, H | C, L, H |
| 38 | 74.48 | 875 | 671 | 531 | methyl-cat-methylpyranomv-3-O-acetgluc [5] | C, L, H | H, L |
| Ethyl-flavanol-methylpyranoanthocyanins | |||||||
| 39 | 61.63 | 847 | 685 | 531 | ethyl-cat-methylpyranomv-3-O-gluc [6] | C, L, H | C, L, H |
| 40 | 71.38 | 847 | 685 | 531 | ethyl-epicat-methylpyranomv-3-O-gluc | - | C, L, H |
| 41 | 82 | 993 | 685 | 531 | ethyl-cat-methylpyranomv-3-O-cumgluc | C, L, H | - |
| Pyranoanthocyanins-flavanols | |||||||
| 42 | 50.0 | 805 | 643 | 491 | pyranomv-3-O-gluc-cat [24] | C, H | - |
| 43 | 68.64 | 805 | 643 | 491 | pyranomv-3-O-gluc-epicat | C, H | - |
| 44 | 76.36 | 951 | 643 | 491 | pyranomv-3-O-coumgluc-cat | H, L | C, L, H |
| Anthocyanin-aldehyde-flavanols | |||||||
| 45 | 55.92 | 809 | 357 | mv-3-O-gluc-ethyl-cat [5] | C, L, H | C, L, H | |
| 46 | 56.6 | 985 | 823 | 661 | mv-3-O-cafglc-propyl-cat | C, L, H | C, L, H |
| 47 | 64.97 | 851 | 357 | mv-3-O-acetylgluc-ethyl-cat | C | - | |
| 48 | 70.79 | 955 | 357 | mv-3-O-coumglc-ethyl-cat | - | C, L | |
| 49 | 80.76 | 955 | 357 | mv-3-O-coumgluc-ethyl-(epi)cat | - | C, L, H | |
| Pyranoanthocyanins-phenol | |||||||
| 50 | 78.23 | 609 | 447 | pyranomv-3-O-gluc-phenol [19,20] | - | C, L, H | |
| 51 | 81.96 | 651 | 447 | pyranomv-3-O-acetgluc-phenol [19,20] | - | C, L, H | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prat-García, S.; Oliveira, J.; del Alamo-Sanza, M.; de Freitas, V.; Nevares, I.; Mateus, N. Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels. Molecules 2021, 26, 64. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010064
Prat-García S, Oliveira J, del Alamo-Sanza M, de Freitas V, Nevares I, Mateus N. Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels. Molecules. 2021; 26(1):64. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010064
Chicago/Turabian StylePrat-García, Samanta, Joana Oliveira, Maria del Alamo-Sanza, Victor de Freitas, Ignacio Nevares, and Nuno Mateus. 2021. "Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels" Molecules 26, no. 1: 64. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010064