Novel D-A-D Fluorescent Dyes Based on 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a Donor Unit for Solution-Processed Organic Light-Emitting-Diodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Photophysical and Electrochemical Properties
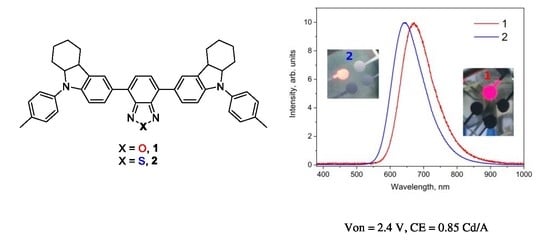
2.3. Photoluminescence Studies
2.4. Theoretical Calculations
2.5. Electroluminescence
- ITO/PEDOT-PSS (50 nm)/poly-TPD (20 nm)/1a (10 nm)/TPBi (20 nm)/LiF (1 nm)/Al (100 nm)—Structure 1;
- ITO/PEDOT-PSS (50 nm)/poly-TPD (20 nm)/1b (10 nm)/TPBi (20 nm)/LiF (1 nm)/Al (100 nm)—Structure 2.
3. Experimental Details
3.1. Materials and Reagents
3.2. Analytical Instruments
3.3. Computational Details
3.4. Device Fabrication and Characterization
3.5. Electrochemical Characterization
3.6. General Procedure for the Preparation of Bis-Substituted Products 1(a–c) under Suzuki Coupling Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qian, G.; Dai, B.; Luo, M.; Yu, D.; Zhan, J.; Zhang, Z.; Ma, D.; Wang, Z.Y. Band gap tunable, donor-acceptor-donor charge-transfer heteroquinoid-based chromophores: Near infrared photoluminescence and electroluminescence. Chem. Mater. 2008, 20, 6208–6216. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Bu, L.; Xie, Z.; Cheng, Y.; Geng, Y.; Wang, L.; Jing, X.; Wang, F. White electroluminescence from a single-polymer system with simultaneous two-color emission: Polyfluorene blue host and side-chain-located orange dopant. Adv. Funct. Mater. 2007, 17, 1917–1925. [Google Scholar] [CrossRef]
- Kostyuchenko, A.S.; Wiosna-Salyga, G.; Kurowska, A.; Zagorska, M.; Luszczynska, B.; Grykien, R.; Glowacki, I.; Fisyuk, A.S.; Domagala, W.; Pron, A. Effect of the electron-accepting centre and solubilising substituents on the redox, spectroscopic and electroluminescent properties of four oxadiazoles and a triazole disubstituted with bithiophene. J. Mater. Sci. 2016, 51, 2274–2282. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Zhang, J.; Tang, W.; Tang, A.; Peng, H.; Xu, Z.; Teng, F.; Wang, Y. Key issues and recent progress of high efficient organic light-emitting diodes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 69–104. [Google Scholar] [CrossRef]
- Deng, D.; Yang, Y.; Zhang, J.; He, C.; Zhang, M.; Zhang, Z.-G.; Zhang, Z.; Li, Y. Triphenylamine-containing linear D-A-D molecules with benzothiadiazole as acceptor unit for bulk-heterojunction organic solar cells. Org. Electron. 2011, 12, 614–622. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.; He, C.; Deng, D.; Li, Y. Triphenylamine-containing D-A-D molecules with (dicyanomethylene)pyran as an acceptor unit for bulk-heterojunction organic solar cells. J. Mater. Chem. 2011, 21, 3768–3774. [Google Scholar] [CrossRef]
- Dutta, G.K.; Patil, S. Solution processable quinoxaline based molecular materials for organic field effect transistors. Org. Electron. 2012, 13, 1266–1276. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Leclère, P.; Surin, M.; Lazzaroni, R.; Lin, T.T.; Dodabalapur, A.; Sellinger, A. Synthesis, characterization and comparative study of thiophene-benzothiadiazole based donor-acceptor-donor (D-A-D) materials. J. Mater. Chem. 2009, 19, 3228–3237. [Google Scholar] [CrossRef]
- Minotto, A.; Haigh, P.A.; Lukasiewicz, L.G.; Lunedei, E.; Gryko, D.T.; Darwazeh, I.; Cacialli, F. Visible light communication with efficient far-red/near-infrared polymer light-emitting diodes. Light Sci. Appl. 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Pramanik, A.; Ahmed, T.; Sahoo, S.K.; Sarkar, P. Superiority of D-A-D over D-A type of organic dyes for the application in dye-sensitized solar cell. Chem. Phys. Lett. 2016, 649, 23–28. [Google Scholar] [CrossRef]
- Patrizi, B.; Cozza, C.; Pietropaolo, A.; Foggi, P.; de Cumis, M.S. Synergistic Approach of Ultrafast Spectroscopy and Molecular Simulations in the Characterization of Intramolecular Charge Transfer in Push-Pull Molecules. Molecules 2020, 25, 430. [Google Scholar] [CrossRef] [Green Version]
- Pazini, A.; Maqueira, L.; Santos, F.S.; Jardim Barreto, A.R.; Carvalho, R.S.; Valente, F.M.; Back, D.; Aucelio, R.Q.; Cremona, M.; Rodembusch, F.S.; et al. Designing highly luminescent aryloxy-benzothiadiazole derivatives with aggregation-induced enhanced emission. Dyes Pigment. 2020, 178, 108377. [Google Scholar] [CrossRef]
- Appalanaidu, E.; Vidya, V.M.; Busireddy, M.R.; Vaidya, J.R.; Chetti, P. Effect of fluorine on optoelectronic properties in DI-A-DII-A-DI type organic molecules: A combined experimental and DFT study. J. Mol. Struct. 2020, 1210, 128019. [Google Scholar] [CrossRef]
- Sudyoadsuk, T.; Chasing, P.; Kaewpuang, T.; Manyum, T.; Chaiwai, C.; Namuangruk, S.; Promarak, V. High efficiency and low efficiency roll-off hole-transporting layer-free solution-processed fluorescent NIR-OLEDs based on oligothiophene-benzothiadiazole derivatives. J. Mater. Chem. C 2020, 8, 5045–5050. [Google Scholar] [CrossRef]
- Qian, G.; Zhong, Z.; Luo, M.; Yu, D.; Zhang, Z.; Wang, Z.Y.; Ma, D. Simple and Efficient Near-Infrared Organic Chromophores for Light-Emitting Diodes with Single Electroluminescent Emission above 1000 nm. Adv. Mater. 2009, 21, 111–116. [Google Scholar] [CrossRef]
- Du, X.; Qi, J.; Zhang, Z.; Ma, D.; Wang, Z.Y. Efficient Non-doped Near Infrared Organic Light-Emitting Devices Based on Fluorophores with Aggregation-Induced Emission Enhancement. Chem. Mater. 2012, 24, 2178–2185. [Google Scholar] [CrossRef]
- Raju, T.B.; Vaghasiya, J.V.; Afroz, M.A.; Soni, S.S.; Iyer, P.K. Influence of m-fluorine substituted phenylene spacer dyes in dye-sensitized solar cells. Org. Electron. 2016, 39, 371–379. [Google Scholar] [CrossRef]
- Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Tanaka, E.; Zhang, L.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole—A new donor building-block in the design of sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A 2020, 391, 112333. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W.H. Organic sensitizers from D-π-A to D-A-π-A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgusa, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Knyazeva, E.A.; Taydakov, I.V.; Mikhalchenko, L.V.; Varaksina, E.A.; Saifutyarov, R.S.; Avetissov, I.C.; Rakitin, O.A. A novel candle light-style OLED with a record low colour temperature. Chem. Commun. 2019, 55, 13354–13357. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dyes Pigment. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Dayneko, S.V.; Rahmati, M.; Pahlevani, M.; Welch, G.C. Solution processed red organic light-emitting-diodes using an N-annulated perylene diimide fluorophore. J. Mater. Chem. C. 2020, 8, 2314–2319. [Google Scholar] [CrossRef]
- Kozma, E.; Mroz, W.; Villafiorita-Monteleone, F.; Galeotti, F.; Andicsova-Eckstein, A.; Catellani, M.; Botta, C. Perylene diimide derivatives as red and deep red-emitters for fully solution processable OLEDs. RSC Adv. 2016, 6, 61175–61179. [Google Scholar] [CrossRef]
- Song, X.; Wang, M.; Kong, L.; Zhao, J. Effects of the acceptor pattern and substitution position on the properties of N-phenyl-carbazolyl based donor-acceptor-donor molecules. RSC Adv. 2017, 7, 18189–18198. [Google Scholar] [CrossRef] [Green Version]
- Bi, X.; Zuo, W.; Liu, Y.; Zhang, Z.; Zeng, C.; Xu, S.; Cao, S. Organic solution-processible electroluminescent molecular glasses for non-doped standard red OLEDs with electrically stable chromaticity. Mater. Res. Bull. 2015, 70, 865–875. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletete, M.; Durocher, G.; Tao, Y.; Leclerc, M. Toward a rational design of poly(2,7-carbazole) derivatives for solar cells. J. Am. Chem. Soc. 2008, 130, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Pilgram, K.; Zupan, M. Bromination of 2,1,3-benzothiadiazoles. J. Heterocycl. Chem. 1970, 7, 629–633. [Google Scholar] [CrossRef]
- Sun, X.; Lei, X.; Hu, Y. Synthesis and characterization of conjugated polymers based on benzoselenadiazole. Asian J. Chem. 2015, 27, 2427–3240. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]








| Entry | Solvent | Dibromide | Temp. (°C) | Time (h) | Yields (%) |
|---|---|---|---|---|---|
| 1 | THF | 2a | 66 | 10 | 1a (25) |
| 2 | THF | 2b | 66 | 10 | 1b (20) |
| 3 | THF | 2c | 66 | 10 | 1c (22) |
| 4 | Dioxane | 2a | 101 | 24 | 1a (85) |
| 5 | Dioxane | 2b | 101 | 24 | 1b (82) |
| 6 | Dioxane | 2c | 101 | 24 | 1c (80) |
| Compound | Ered (vs Fc/Fc+) 1, V | ELUMO 2, eV | Eox (vs Fc/Fc+) 1, V | EHOMO 2, eV | Eg 3, eV |
|---|---|---|---|---|---|
| 1a | −1.80 | −3.30 | 0.25 | −5.35 | 2.05 |
| 1b | −1.88 | −3.22 | 0.26 | −5.36 | 2.14 |
| 1c | −1.59 | −3.51 | 0.22 | −5.32 | 1.81 |
| Solvent | λabs nm | εmax × 104 mol 1−1 cm−1 | λem nm | FWHM nm | Δν Cm−1 | Φ % | Kr × 107 S−1 | Knr × 108 S−1 | τ ns | f | μeg Debye |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cyclohexane | 485 | 10 | 594 | 90 | 3784 | 56 | - | - | - | 0.68 | 2.1 |
| CHCl3 | 437 | 22 | 610 | 121 | 6490 | 34 | 9.1 | 1.8 | 3.7 | 0.82 | 8.1 |
| AcOEt | 470 | 6 | 660 | 132 | 6125 | 23 | 3.3 | 1.1 | 6.9 | 0,78 | 8.0 |
| THF | 470 | 11 | 662 | 126 | 6170 | 21 | 3.1 | 1.2 | 6.8 | 0.72 | 7.8 |
| DMSO | 484 | 12 | 761 | 160 | 7520 | 11 | 5.5 | 4.5 | 2.0 | 0.50 | 6.0 |
| Comp. | ELUMO eV | EHOMO eV | λabs a nm | λabs b nm | S1 c nm | Major Contribution | λem f nm | λem g nm | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | −0.34 | −6.18 | 578.5 | 480 | 543 | 1.07/0.92 | 3.02/9.67 | H→ L 93% H-2→ L 5% | 551.8 | 660 |
| 1b | −0.95 | −6.15 | 564.3 | 470 | 559 | 0.83/0.71 | 4.88/7.80 | H→ L 93% H-2→ L 5% | 715 | 662 |
| 1c | −1.02 | −6.14 | 590.8 | 495 | 567 | 0.68/0.83 | 5.41/9.33 | H→ L 93% H-2→ L 6% | 765 | 705 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korshunov, V.M.; Mikhailov, M.S.; Chmovzh, T.N.; Vashchenko, A.A.; Gudim, N.S.; Mikhalchenko, L.V.; Taydakov, I.V.; Rakitin, O.A. Novel D-A-D Fluorescent Dyes Based on 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a Donor Unit for Solution-Processed Organic Light-Emitting-Diodes. Molecules 2021, 26, 2872. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102872
Korshunov VM, Mikhailov MS, Chmovzh TN, Vashchenko AA, Gudim NS, Mikhalchenko LV, Taydakov IV, Rakitin OA. Novel D-A-D Fluorescent Dyes Based on 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a Donor Unit for Solution-Processed Organic Light-Emitting-Diodes. Molecules. 2021; 26(10):2872. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102872
Chicago/Turabian StyleKorshunov, Vladislav M., Maxim S. Mikhailov, Timofey N. Chmovzh, Andrey A. Vashchenko, Nikita S. Gudim, Lyudmila V. Mikhalchenko, Ilya V. Taydakov, and Oleg A. Rakitin. 2021. "Novel D-A-D Fluorescent Dyes Based on 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a Donor Unit for Solution-Processed Organic Light-Emitting-Diodes" Molecules 26, no. 10: 2872. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102872