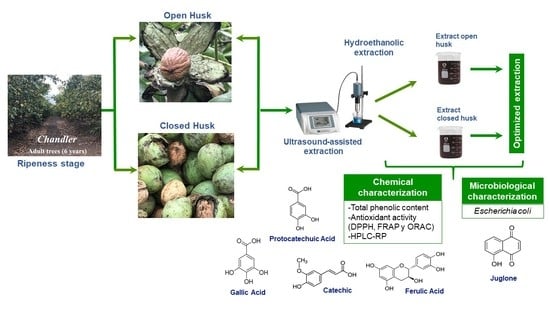
Dependence of the Ripeness Stage on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts from Industrial By-Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Walnut Green Husks
2.1.1. Proximal Analysis
2.1.2. Antioxidant Capacity
2.1.3. Identification of Phenolic Compound Profiles
2.2. Optimization of the Extraction Process of the Walnut Green Husk
2.3. Evaluation of the Antioxidant and Antimicrobial Capacity of the Optimized Extracts of Walnut Green Husk
2.3.1. Antioxidant Capacity
2.3.2. Antimicrobial Capacity
3. Materials and Methods
3.1. Samples
3.2. Walnut Green Husk Drying
3.3. Proximal Characterization
3.4. Control Extraction
3.5. Ultrasound-Assisted Extractions
3.6. Identification of Phenolic Compounds by HPLC-DAD-FLD
3.7. Determination of Total Phenolic Content
3.8. Antioxidant Activity
3.8.1. DPPH Radical Scavenging Assay
3.8.2. Ferric Reducing Antioxidant Power (FRAP)
3.8.3. Oxygen Radical Absorbance Capacity (ORAC)
3.9. Experimental Design
3.10. Kinetics of Bacterial Growth
Kinetic Model
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ProChile Ministerio de Relaciones Exteriores. 2020. Available online: https://www.prochile.gob.cl/noticia/numero-record-de-empresas-marca-la-participacion-de-chile-en-la-25-version-de-gulfood-en-dubai/#:~:text=A%20nivel%20mundial%2C%20Chile%20es,de%20Estados%20Unidos%20y%20M%C3%A9xico (accessed on 20 March 2021).
- Chilenut. Asociación de Productores y Exportadores de Nueces. 2017. Available online: http://www.chilenut.cl/index.php?seccion=nuez-de-nogal (accessed on 15 December 2019).
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential, and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, B.; Jiang, Y.; Liu, Z.; Liu, Y.; Wang, X.; Kuang, H. Studies on cytotoxic activity against HepG-2 cells of naphthoquinones from walnut green husks of Juglans mandshurica Maxim. Molecules 2015, 20, 15572–15588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Dominguez-Rodriguez, G.; Castro-Puyana, M.; Marina, M.L. Polyphenols Analysis and Related Challenges. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing Inc.: Sawston, UK, 2018; pp. 117–220. [Google Scholar]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Jakopic, J.; Veberic, R. Extraction of phenolic compounds from green walnut fruits in different solvents. Acta Agric. Slov. 2009, 93, 11. [Google Scholar] [CrossRef] [Green Version]
- Cosmulescu, S.N.; Trandafir, I.; Achim, G.; Mihai, B.O.T.U.; Baciu, A.; Gruia, M. Phenolics of green husk in mature walnut fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 53–56. [Google Scholar]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crop. Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Vergara-Castro, M.; Jara-Quezada, J.; Caballero-Valdés, E.; Müller-Pavez, A.; Zúñiga-Hansen, M.E.; Altamirano, C. Polyphenolic extracts of walnut (Juglans regia L.) green husk containing juglone inhibit the growth of HL-60 cells and induce apoptosis. Electron. J. Biotechnol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.; Ferreira, O.; Barrosa, L.; Ferreira, I.C. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Yang, Z.; Ding, Z. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Ufsenik, V.; Osterc, G.; Petkovšek, M.M.; Štefančič, M.; Veberič, R.; Colarič, M.; Solar, A.; Stampar, F. The Involvement of Phenolic Compounds in the Metabolism of Fruit Trees; Razprave IV, razreda SAZU: Ljubljana, Slovenska, 2004; pp. 187–204. [Google Scholar]
- Jay-Allemand, C.; Bruant, B.; Burtin, P.; Fady, B.; Lefevre, F.; Germain, E. Genetic of Phenolic Compounds in Walnut: Qualitative and Quantitative Variations among Cultivars. Acta Hortic. 1999, 544, 73–81. [Google Scholar] [CrossRef]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids, and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienzle, S.; Sruamsiri, P.; Carle, R.; Sirisakulwat, S.; Spreer, W.; Neidhart, S. Harvest maturity specification for mango fruit (Mangifera indica L. ‘Chok Anan’) in regard to long supply chains. Postharvest Biol. Technol. 2011, 61, 41–55. [Google Scholar] [CrossRef]
- Lemus, G. (Ed.) Cosecha. In El Nogal en Chile; Colección de libros INIA N°6; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2001; p. 224. [Google Scholar]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- INTA. Portal Antioxidante. 2013. Available online: http://www.portalantioxidantes.com/orac-base-de-datos-actividad-antioxidante-y-contenido-de-polifenoles-totales-en-frutas/ (accessed on 5 December 2019).
- Laroze, L.; Soto, C.; Zúñiga, M.E. Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electron. J. Biotechnol. 2010, 13, 11–12. [Google Scholar] [CrossRef]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur–cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Fukuda, T. Walnut Polyphenols: Their Structures and Functions. In Tree Nuts: Composition, Phytochemicals, and Health Effects; Alasalvar, C., Shahidi, F., Eds.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2008; pp. 305–320. [Google Scholar]
- Zhang, Y.; Cui, Y.; Zhu, J.; Li, H.; Mao, J.; Jin, X.; Lu, J. The anti-tumor effect and biological activities of the extract JMM6 from the stem-barks of the Chinese Juglans mandshurica Maxim on human hepato & cell line BEL-7402. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 258–269. [Google Scholar] [PubMed] [Green Version]
- Akanbi, T.O.; Marshall, S.N.; Barrow, C.J. Polydatin-fatty acid conjugates are effective antioxidants for stabilizing omega 3-containing bulk fish oil and fish oil emulsions. Food Chem. 2019, 301, 125297. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Ma, Q.H.; Xu, Y. Characterization of a caffeic acid 3-O-methyltransferase from wheat and its function in lignin biosynthesis. Biochimie 2008, 90, 515–524. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Macarulla, M.T.; Rotches-Ribalta, M.; Boto-Ordóñez, M.; Urpi-Sarda, M.; Rodríguez, V.M.; Portillo, M.P. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J. Agric. Food Chem. 2012, 60, 4833–4840. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, C. The emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. 2021. Available online: https://www.who.int/es/news-room/fact-sheets/detail/e-coli (accessed on 4 May 2021).
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Hamilton-Miller, J.M.; Hara, Y.; Nagaoka, Y.; Kumagai, A.; Uesato, S.; Taylor, P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.; Nunan, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Radix, P.; Bastien, C.; Jay-Allemand, C.; Charlot, G.; Seigle-Murandi, F. The influence of soil nature on polyphenols in walnut tissues. A possible explanation of differences in the expression of walnut blight. Agronomie 1998, 18, 627–637. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Campbell, B.C. Regulation of aflatoxin production by naphthoquinones of walnut (Juglans regia). J. Agric. Food Chem. 2000, 48, 4418–4421. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using Fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Pilaquinga, F.; Amaguaña, D.; Morey, J.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Fernández, L.; Espinosa-Montero, P.; Jara-Negrete, E.; Meneses, L.; et al. Synthesis of silver nanoparticles using aqueous leaf extract of Mimosa albida (Mimosoideae): Characterization and antioxidant activity. Materials 2020, 13, 503. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, P.H.; De la Vara, R. Análisis y Diseño de Experimentos, 3rd ed.; Mc Graw Hill: Mexico City, Mexico, 2012; pp. 343–367. [Google Scholar]
- Celis-Cofré, D.; Azócar, M.I.; Enrione, J.; Páez, M.; Matiacevich, S. Influence of glassy or rubbery state on the antimicrobial activity of chitosan-gelatin films. J. Food Res. 2012, 1, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Van Boekel, M.A. Kinetic modeling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]




| Percent (%) | Open Husk (OH) | Closed Husk (CH) |
|---|---|---|
| Moisture | 11.716 a ± 0.147 | 14.074 b ± 0.679 |
| Proteins | 4.694 a ± 0.190 | 4.452 a ± 0.129 |
| Lipids | 1.385 a ± 0.788 | 1.610 a ± 0.129 |
| Ashes | 18.515 a ± 0.949 | 17.509 a ± 1.393 |
| Crude fiber | 44.700 a ± 0.018 | 44.800 a ± 0.027 |
| Non-nitrogen extract | 18.918 a ± 0.051 | 17.243 a ± 1.806 |
| Polyphenol | Detection | Control Extraction | Optimized Extraction | |||
|---|---|---|---|---|---|---|
| DAD (nm) | FLD (nm) | OH | CH | OH | CH | |
| Gallic acid | 272 | - | ✓ | ✓ | ✓ | ✓ |
| Protocatechuic acid | 260–290 | - | ✓ | ✓ | ✓ | ✓ |
| Catechin | 278 | 280–318 | ✓ | ✓ | - | ✓ |
| Caffeic acid | 322 | - | ✓ | - | - | - |
| Ferulic acid | 322 | 332–445 | ✓ | ✓ | ✓ | ✓ |
| Polydatin | 306–318 | 320–395 | ✓ | - | - | - |
| Hesperetin | 284 | - | - | - | - | - |
| Resveratrol | 306–318 | 323–390 | - | - | - | - |
| Quercetin | 254–370 | - | ✓ | ✓ | - | - |
| Myricetin | 250–370 | - | - | - | - | - |
| Kaempferol | 270–366 | - | ✓ | ✓ | - | - |
| Hesperidin | 288 | - | ✓ | ✓ | - | - |
| Juglone | 235–275 | - | - | - | ✓ | ✓ |
| Independent Variables | Responses Variables | |||||
|---|---|---|---|---|---|---|
| Run | X1: Solid/Solvent Ratio (g/mL) | X2: Ethanol/Water Ratio (v/v) | Y1: TPC (mg GAE/g Sample dw) | Y2: DPPH (mg Trolox/g Sample dw) | Y3: FRAP (mg FeSO4/g Sample dw) | Y4: ORAC (µmol Trolox/g Sample dw) |
| 1 | 1/20 | 50/50 | 105.99 ± 0.85 | 152.3 ± 3.0 | 102.4 ± 2.7 | 130 ± 1.4 |
| 2 | 1/30 | 75/25 | 102.21 ± 0.71 | 199.4 ± 2.8 | 123.7 ± 3.4 | 182.3 ± 2.5 |
| 3 | 1/10 | 25/75 | 31.17 ± 0.18 | 75.4 ± 1.1 | 65.2 ± 0.2 | 78.4 ± 0.86 |
| 4 | 1/10 | 75/25 | 45.95 ± 0.10 | 91.6 ± 0.6 | 83.1 ± 1.4 | 121.81 ± 0.64 |
| 5 | 1.4/40 | 50/50 | 67.02 ± 0.12 | 153.0 ± 0.8 | 78.4 ± 1.7 | 142.4 ± 4.4 |
| 6 | 1/30 | 25/75 | 57.07 ± 0.40 | 86.6 ± 1.9 | 59.6 ± 0.9 | 102.2 ± 0.86 |
| 7 | 1/20 | 50/50 | 106.01 ± 0.85 | 153.8 ± 1.5 | 96.6 ± 0.2 | 137.07 ± 0.06 |
| 8 | 1.4/14 | 50/50 | 50.12 ± 0.04 | 89.3 ± 0.1 | 80.5 ± 2.0 | 102.95 ± 0.38 |
| 9 | 1/20 | 35/65 | 65.05 ± 0.10 | 127.8 ± 0.5 | 81.2 ± 3.3 | 154.83 ± 1.08 |
| 10 | 1/20 | 65/35 | 82.43 ± 0.02 | 166.4 ± 0.9 | 120.3 ± 3.4 | 167.3 ± 0.84 |
| Extract (µg/mL) | Kinetic Parameters | I (%) | RMS (%) | |||
|---|---|---|---|---|---|---|
| As (adim) | µmax (h−1) | λ (h) | ||||
| Escherichia coli | 0.52 a ± 0.02 | 0.09 a ± 0.002 | 1.74 b ± 0.06 | --- | 5.0 | |
| OH | 16 | 0.26 b ± 0.05 | 0.03 b ± 0.01 | 1.57 b ± 0.14 | 44.4 ± 3.7 | 5.0 |
| 32 | 0.25 bc ± 0.01 | 0.03 bc ± 0.004 | 1.25 b ± 0.21 | 51.6 ± 2.2 | 7.0 | |
| 48 | 0.22 bcd ± 0.05 | 0.013 efg ± 0.005 | 3.90 c ± 0.09 | 63.4 ± 2.0 | 12.0 | |
| 64 | 0.22 bcd ± 0.03 | 0.009 fgh ± 0.003 | 3.70 c ± 0.60 | 62.0 ± 0.1 | 9.8 | |
| 80 | 0.04 f ± 0.005 | 0.004 gh ± 0.001 | 5.00 d ± 0.04 | 78.4 ± 7.5 | 8.7 | |
| 96 | 0.04 g ± 0.005 | 0.003 h ± 0.001 | 3.35 c ± 0.57 | 93.2 ± 1.0 | 8.1 | |
| CH | 16 | 0.26 b ± 0.02 | 0.03 bc ± 0.004 | 1.62 b ± 0.15 | 50.8 ± 3.2 | 5.3 |
| 32 | 0.25 bc ± 0.01 | 0.024 cd ± 0.005 | 1.55 b ± 0.10 | 52.5 ± 1.6 | 6.2 | |
| 48 | 0.23 bcd ± 0.03 | 0.018 de ± 0.001 | 2.22 b ± 1.16 | 58.7 ± 2.0 | 7.4 | |
| 64 | 0.19 cde ± 0.01 | 0.016 ef ± 0.003 | 1.95 b ± 1.23 | 63.1 ± 2.6 | 9.4 | |
| 80 | 0.19 de ± 0.03 | 0.013 ef ± 0.004 | 1.92 b ± 0.05 | 66.1 ± 4.7 | 6.3 | |
| 96 | 0.15 ef ± 0.03 | 0.01 fg ± 0.001 | 0.00 a ± 0.00 | 68.9 ± 2.4 | 10.0 | |
| Independent Variables | Level | ||
| Low (−1) | Medium (0) | High (1) | |
| X1: solid/solvent ratio (g/mL) | 1:10 | 1:20 | 1:30 |
| X2: ethanol/water ratio (v/v) | 25/75 | 50/50 | 75/25 |
| Responses Variables | Goal | ||
| Y1: TPC (mg GAE/g sample dw) | Maximize | ||
| Y2: DPPH (mg Trolox/g sample dw) | |||
| Y3: FRAP (mg FeSO4/g sample dw) | |||
| Y4: ORAC (µmol Trolox/g sample dw) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Madrid, D.; Gutiérrez-Cutiño, M.; Pozo-Martínez, J.; Zúñiga-López, M.C.; Olea-Azar, C.; Matiacevich, S. Dependence of the Ripeness Stage on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts from Industrial By-Products. Molecules 2021, 26, 2878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102878
Soto-Madrid D, Gutiérrez-Cutiño M, Pozo-Martínez J, Zúñiga-López MC, Olea-Azar C, Matiacevich S. Dependence of the Ripeness Stage on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts from Industrial By-Products. Molecules. 2021; 26(10):2878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102878
Chicago/Turabian StyleSoto-Madrid, Daniela, Marlen Gutiérrez-Cutiño, Josué Pozo-Martínez, María Carolina Zúñiga-López, Claudio Olea-Azar, and Silvia Matiacevich. 2021. "Dependence of the Ripeness Stage on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts from Industrial By-Products" Molecules 26, no. 10: 2878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102878