Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid
Abstract
:1. Introduction
2. Results
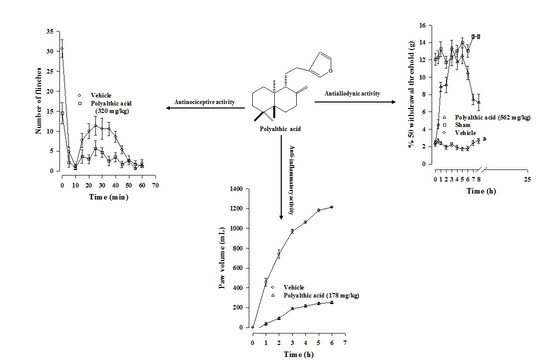
2.1. Antinociceptive Effect of Polyalthic Acid
2.2. Possible Mechanisms of the Antinociceptive Effect of Polyalthic Acid
2.3. Antiallodynic Effect of Polyalthic Acid on Rats with an L5/L6 Spinal Nerve Ligation
2.4. Antihyperalgesic Effect of Polyalthic Acid and Naproxen
2.5. Synergistic Interaction between Polyalthic Acid and Naproxen
2.6. Anti-Inflammatory Effect of Polyalthic Acid
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Formalin Test
4.4. L5/L6 Spinal Nerve Ligation
4.5. Thermal Hyperalgesia
4.6. Isobologram
4.7. Carrageenan-Induced Paw Edema
4.8. Experimental Design
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Williams, A.C.D.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Olivieri, J.; Allison, J.J.; Gaffo, A.; Juarez, L.; Kovac, S.H.; Person, S.; Saag, K.G. A group randomized trial to improve safe use of nonsteroidal anti-inflammatory drugs. Am. J. Manag. Care 2005, 11, 537–543. [Google Scholar]
- Simon, L.S. Nonsteroidal anti-inflammatory drugs and their risk: A story still in development. Arthritis Res. Ther. 2013, 15, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.L.; Viappiani, S.; Bolla, M. Cyclooxygenase-inhibiting nitric oxide donators for osteoarthritis. Trends Pharmacol. Sci. 2009, 30, 112–117. [Google Scholar] [CrossRef]
- Carlo, P. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar]
- Reza, T. Risk of Cardiovascular Events and Cyclooxygenase-2 Inhibitors. Vasc. Health. Risk. Manag. 2006, 2, 95. [Google Scholar]
- Babu, T.H.; Manjulatha, K.; Kumar, G.S.; Hymavathi, A.; Tiwari, A.K.; Purohit, M.; Rao, J.M.; Babu, K.S. Gastroprotective flavonoid constituents from Oroxylum indicum Vent. Bioorganic Med. Chem. Lett. 2010, 20, 117–120. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Frade, R.F.M.; Afonso, C.A.M. Isolation, Chemical, and Biotransformation Routes of Labdane-type Diterpenes. Chem. Rev. 2011, 111, 4418–4452. [Google Scholar] [CrossRef]
- Reyes-Trejo, B.; Sánchez-Mendoza, M.E.; Becerra-García, A.A.; Cedillo-Portugal, E.; Castillo-Henkel, C.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer diterpenoid from Croton reflexifolius: Role of nitric oxide, prostaglandins and sulfhydryls. J. Pharm. Pharmacol. 2008, 60, 931–936. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; De La Rosa, L.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Arrieta, J. Polyalthic Acid Isolated from Croton reflexifolius has Relaxing Effect in Guinea Pig Tracheal Smooth Muscle. Pharm. Biol. 2008, 46, 800–807. [Google Scholar] [CrossRef]
- Huang, D.; Qing, S.; Zeng, G.; Wang, Y.; Guo, H.; Tan, J.; Zhou, Y. Lipophilic components from Fructus Viticis Negundo and their anti-tumor activities. Fitoterapia 2013, 86, 144–148. [Google Scholar] [CrossRef]
- Borges, C.H.G.; Cruz, M.G.; Carneiro, L.J.; Da Silva, J.J.M.; Bastos, J.K.; Tavares, D.C.; De Oliveira, P.F.; Rodrigues, V.; Veneziani, R.C.S.; Parreira, R.L.T.; et al. Copaifera duckei Oleoresin and Its Main Nonvolatile Terpenes: In Vitro Schistosomicidal Properties. Chem. Biodivers. 2016, 13, 1348–1356. [Google Scholar] [CrossRef]
- Bardají, D.K.; da Silva, J.J.; Bianchi, T.C.; de Souza, E.D.; de Oliveira, P.F.; Leandro, L.F.; Rogez, H.L.; Venezianni, R.C.; Ambrosio, S.R.; Tavares, D.C.; et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, B.; Zelazowska, E.; Raybourne, R.B.; Cizza, G.; Sternberg, E.M. Inflammatory responses to carrageenan injection in LEW/N and F344/N rats: LEW/N rats show sex- and age-dependent changes in inflammatory reactions. Neuroimmunomodulation 1996, 3, 93–101. [Google Scholar] [CrossRef]
- Tall, J.M.; Crisp, T. Effects of gender and gonadal hormones on nociceptive responses to intraplantar carrageenan in the rat. Neurosci. Lett. 2004, 354, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Ozaki, G.; McCumber, D.; Rathbun, M.; Svensson, C.; Malkmus, S.; Yaksh, M.C. An automated flinch detecting system for use in the formalin nociceptive bioassay. J. Appl. Physiol. 2001, 90, 2386–2402. [Google Scholar] [CrossRef]
- Vincler, M.; Maixner, W.; Vierck, C.J.; Light, A.R. Estrous cycle modulation of nociceptive behaviors elicited by electrical stimulation and formalin. Pharmacol. Biochem. Behav. 2001, 69, 315–324. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Albonetti, M.E.; Carli, G. Behavioural effects of different intensities of formalin pain in rats. Physiol. Behav. 1995, 58, 603–610. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Sacerdote, P.; Albonetti, M.E.; Carli, G. Sex-related effects on behaviour and beta-endorphin of different intensities of formalin pain in rats. Brain Res. 1995, 699, 242–249. [Google Scholar] [CrossRef]
- Caram-Salas, N.L.; Reyes-García, G.; Bartoszyk, G.D.; Araiza-Saldaña, C.I.; Ambriz-Tututi, M.; Rocha-González, H.I.; Arreola-Espino, R.; Cruz, S.L.; Granados-Soto, V. Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur. J. Pharmacol. 2007, 573, 75–83. [Google Scholar] [CrossRef]
- Lima, F.O.; Souza, G.R.; Verri, W.A.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: Involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain 2010, 151, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Duarte, I.D. Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K+ channels in the rat PGE2 -induced hyperalgesic paw. Br. J. Pharmacol. 2001, 134, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, F.Q.; Teixeira, M.M.; Ferreira, S.H. Pharmacological modulation of secondary mediator systems-cyclic AMP and cyclic GMP-on inflammatory hyperalgesia. Br. J. Pharmacol. 1999, 127, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, M.; Treadwell, J.R.; Tregear, S.J.; Coates, V.H.; Wiffen, P.J.; Akafomo, C.; Schoelles, K.M.; Chou, R. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010, 2010, CD006605. [Google Scholar] [CrossRef] [PubMed]
- Meert, T.F.; Vermeirsch, H.A. A preclinical comparison between different opioids: Antinociceptive versus adverse effects. Pharmacol. Biochem. Behav. 2005, 80, 309–326. [Google Scholar] [CrossRef]
- Hashemi, M.; Karami, M.; Zarrindast, M.R.; Sahebgharani, M. Role of nitric oxide in the rat hippocampal CA1 in morphine antinociception. Brain Res. 2010, 1313, 79–88. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Duarte, I.D.; Lorenzetti, B.B. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991, 201, 121–122. [Google Scholar] [CrossRef]
- Florentino, I.F.; Galdino, P.M.; De Oliveira, L.P.; Silva, D.P.; Pazini, F.; Vanderlinde, F.A.; Lião, L.M.; Menegatti, R.; Costa, E.A. Involvement of the NO/cGMP/KATP pathway in the antinociceptive effect of the new pyrazole 5-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole (LQFM-021). Nitric Oxide. 2015, 47, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef] [Green Version]
- Cunha, T.M.; Souza, G.R.; Domingues, A.C.; Carreira, E.U.; Lotufo, C.M.; Funez, M.I.; Verri, W.A.; Cunha, F.Q.; Ferreira, S.H. Stimulation of Peripheral Kappa Opioid Receptors Inhibits Inflammatory Hyperalgesia via Activation of the PI3Kγ/AKT/nNOS/NO Signaling Pathway. Mol. Pain 2012, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Duggan, K.C.; Walters, M.J.; Musee, J.; Harp, J.M.; Kiefer, J.R.; Oates, J.A.; Marnett, L.J. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J. Biol. Chem. 2010, 285, 34950–34959. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, H.; Kawahara, T. Nonsteroidal Anti-Inflammatory Drugs and Prostatic Diseases. BioMed. Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Robich, M.P.; Chu, L.M.; Feng, J.; Burgess, T.A.; Laham, R.J.; Bianchi, C.; Sellke, F.W. Effects of selective cyclooxygenase-2 and nonselective cyclooxygenase inhibition on ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2010, 140, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, M.I.; González-García, M.P.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Aguilar-Robles, P. Synergistic effect of the interaction between naproxen and citral on inflammation in rats. Phytomedicine 2010, 18, 74–79. [Google Scholar] [CrossRef]
- Wu, X.; Xie, J.; Qiu, L.; Zou, L.; Huang, Y.; Xie, Y.; Xu, H.; He, S.; Zhang, Q. The anti-inflammatory and analgesic activities of the ethyl acetate extract of Viburnum taitoense Hayata. J. Ethnopharmacol. 2021, 6, 113742. [Google Scholar] [CrossRef]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R473–R487. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Wheeler-Aceto, H.; Cowan, A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 1991, 104, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Ortega-Varela, L.F.; Herrera, J.E.; Caram-Salas, N.L.; Rocha-González, H.I.; Granados-Soto, V. Isobolographic Analyses of the Gabapentin-Metamizol Combination after Local Peripheral, Intrathecal and Oral Administration in the Rat. Pharmacology 2007, 79, 214–222. [Google Scholar] [CrossRef]
- Tallarida, R. Calculations for Combination Drug Analysis. In Drug Synergism and Dose-Effect Data Analysis; Chapman and Hall/CRC: Boca Raton, FL, USA, 2000; pp. 57–73. [Google Scholar]
- Winder, C.W.; Wax, J.; Been, M.A. Rapid foot volume measurements on unanesthetized rats, and the question of a phe-nylbutazone effect on anaphylactoid edema. Arch. Int. Pharmacodyn. Ther. 1957, 112, 174–187. [Google Scholar] [PubMed]








| Combination | Oral Dose (mg/kg) | ||
|---|---|---|---|
| Polyalthic Acid | Naproxen | Total Dose in the Combination | |
| 1 | 3.2 | 0.6 | 3.8 |
| 2 | 6.4 | 1.3 | 7.7 |
| 3 | 12.7 | 2.5 | 15.2 |
| 4 | 25.4 | 5.0 | 30.4 |
| 5 | 50.8 | 10 | 60.8 |
| Drug | ED50 (mg/kg) |
|---|---|
| Naproxen | 20.1 ± 4.8 |
| Polyalthic acid | 101.6 ± 30.9 |
| Theoretical ED50 of the polyalthic acid–naproxen combination | 60.9 ± 15.7 CI (32.4–114.3) |
| Experimental ED50 of the polyalthic acid-–naproxen combination | 2.4 ± 0.02 * CI (0.9–6.6) |
| Interaction index (γ) | 0.04 ± 0.02 CI (0.02–0.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Silverio, J.; Sánchez-Mendoza, M.E.; Rocha-González, H.I.; Reyes-García, J.G.; Flores-Murrieta, F.J.; López-Lorenzo, Y.; Quiñonez-Bastidas, G.N.; Arrieta, J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules 2021, 26, 2921. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102921
Rodríguez-Silverio J, Sánchez-Mendoza ME, Rocha-González HI, Reyes-García JG, Flores-Murrieta FJ, López-Lorenzo Y, Quiñonez-Bastidas GN, Arrieta J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules. 2021; 26(10):2921. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102921
Chicago/Turabian StyleRodríguez-Silverio, Juan, María Elena Sánchez-Mendoza, Héctor Isaac Rocha-González, Juan Gerardo Reyes-García, Francisco Javier Flores-Murrieta, Yaraset López-Lorenzo, Geovanna Nallely Quiñonez-Bastidas, and Jesús Arrieta. 2021. "Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid" Molecules 26, no. 10: 2921. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102921