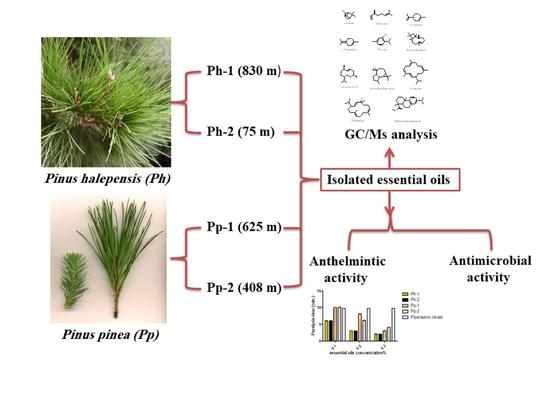
Impact of Altitudinal Variation on the Phytochemical Profile, Anthelmintic and Antimicrobial Activity of Two Pinus Species
Abstract
:1. Introduction
2. Results
2.1. GC/MS Analysis of the Essential Oil
2.2. In Vitro Anthelmintic Activity of the Essential Oils
2.3. In Vitro Antimicrobial Activity of the Essential Oils
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oils Isolation
4.3. GC/MS Analyses
4.4. Identification of Essential Oil Components
4.5. In Vitro Anthelmintic Activity of the Essential Oils
4.6. In Vitro Antimicrobial Activity of the Essential Oils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dkhil, M.A.; Zreiq, R.; Hafiz, T.A.; Mubaraki, M.A.; Sulaiman, S.; Algahtani, F.; Abdel-Gaber, R.; Al-Shaebi, E.M.; Al-Quraishy, S. Anthelmintic and antimicrobial activity of Indigofera oblongifolia leaf extracts. Saudi J. Biol. Sci. 2020, 27, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Schiller, G. Therapeutic use of Aleppo pine (Pinus halepensis Mill.). In Medicinal and Aromatic Plants of the Middle-East; Springer: Dordrecht, The Netherlands, 2014; pp. 215–224. [Google Scholar]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Burhan, A.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Fekih, N.; Allali, H.; Merghache, S.; Chaïb, F.; Merghache, D.; El Amine, M.; Djabou, N.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans Pharmacognosy, 15th ed.; Saunders, W.B., Ed.; Elsevier: Eidenburg, UK; London, UK; New York, NY, USA; Philadelphia, PA, USA; St. Louis, MO, USA; Sydney, Australia; Toronto, ON, Canada, 2002. [Google Scholar]
- Ashmawy, N.A.; Al Farraj, D.A.; Salem, M.Z.; Elshikh, M.S.; Al-Kufaidy, R.; Alshammari, M.K.; Salem, A.Z. Potential impacts of Pinus halepensis Miller trees as a source of phytochemical compounds: Antibacterial activity of the cones essential oil and n-butanol extract. Agrofor. Syst. 2018, 94, 1403–1413. [Google Scholar] [CrossRef]
- Mohareb, A.; Kherallah, I.; Badawy, M.; Salem, M.; Yousef, H. Chemical composition and activity of bark and leaf extracts of Pinus halepensis and Olea europaea grown in AL-Jabel AL-Akhdar region, Libya against some plant phytopathogens. J. Appl. Biotechnol. Bioeng. 2017, 3, 331–342. [Google Scholar]
- Nam, A.M.; Tomi, F.; Gibernau, M.; Casanova, J.; Bighelli, A. Composition and chemical variability of the needle oil from Pinus halepensis growing in Corsica. Chem. Biodivers. 2016, 13, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Halloum, B.; Shakya, A.K.; Elagbar, Z.A.; Naik, R.R. GC-MS Analysis and Biological activity of Essential Oil of Fruits, Needles and Bark of Pinus pinea grown wildly in Jordan. Acta Pol. Pharm.-Drug Res. 2019, 76, 825–831. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Gad, H.; Al-Sayed, E.; Ayoub, I. Phytochemical discrimination of Pinus species based on GC–MS and ATR-IR analyses and their impact on Helicobacter pylori. Phytochem. Anal. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hamrouni, L.; Hanana, M.; Amri, I.; Romane, A.E.; Gargouri, S.; Jamoussi, B. Allelopathic effects of essential oils of Pinus halepensis Miller: Chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Plant Prot. 2015, 48, 145–158. [Google Scholar] [CrossRef]
- Khouja, M.; Alves, R.C.; Melo, D.; Costa, A.S.; Nunes, M.A.; Khaldi, A.; Oliveira, M.B.P.; Messaoud, C. Morphological and chemical differentiation between Tunisian populations of Pinus halepensis Mill. Pinus brutia Ten. and Pinus pinaster Ait. Chem. Biodivers. 2021. [Google Scholar] [CrossRef] [PubMed]
- Djerrad, Z.; Kadik, L.; Djouahri, A. Chemical variability and antioxidant activities among Pinus halepensis Mill. essential oils provenances, depending on geographic variation and environmental conditions. Ind. Crop. Prod. 2015, 74, 440–449. [Google Scholar] [CrossRef]
- Mohareb, A.S.; Kherallah, I.; Badawy, M.E.; Salem, M.; Faraj, H. Chemical composition and antibacterial activity of essential oils isolated from leaves of different woody trees grown in Al-Jabel al-Akhdar region, Libya. Alex. Sci. Exch. J. 2016, 37, 358–371. [Google Scholar]
- Djerrad, Z.; Djouahri, A.; Kadik, L. Variability of Pinus halepensis Mill. Essential oils and their antioxidant activities depending on the stage of growth during vegetative cycle. Chem. Biodivers. 2017, 14, e1600340. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Mendes, M.D.; Lima, A.S.; Barbosa, P.M.; Ascensão, L.; Barroso, J.G.; Pedro, L.G.; Mota, M.M.; Figueiredo, A.C. Pinus halepensis, Pinus pinaster, Pinus pinea and Pinus sylvestris essential oils chemotypes and monoterpene hydrocarbon enantiomers, before and after inoculation with the pinewood nematode Bursaphelenchus xylophilus. Chem. Biodivers. 2017, 14, e1600153. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Belmehdi, O.; Abrini, J.; Dakka, N.; Bakri, Y. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019, 12, 117. [Google Scholar] [CrossRef]
- Haichour, R.; Ramdani, M.; Lograda, T.; Chalard, P.; Figueredo, G. Chemical composition and antimicrobial activity of Pinus halepensis from Algeria. Biodiversitas 2020, 21, 4345–4360. [Google Scholar]
- Maggiore, M.A.; Albanese, A.A.; Gende, L.B.; Eguaras, M.J.; Denegri, G.M.; Elissondo, M.C. Anthelmintic effect of Mentha spp. essential oils on Echinococcus granulosus protoscoleces and metacestodes. Parasitol. Res. 2012, 110, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; El-Jalel, L.; Yousif, M.; Gonaid, M. Altitude impact on the chemical profile and biological activities of Satureja thymbra L. essential oil. BMC Complementary Med. Ther. 2020, 20, 186. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Mihajilov-Krstev, T.; Stojanović-Radić, Z.Z.; Cvetković, V.J.; Mitrović, T.L.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal ac-tivity. Ind. Crop. Prod. 2018, 111, 55–62. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Publ, A., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Dkhil, M.A.; Thagfan, F.A.; Abdel-moniem, S.H.; Al-Shaebi, E.M.; Abdel-Gaber, R.; Al-Quraishy, S. Anthelmintic, anticoccidial and antioxidant activity of Salvadora persica root extracts. Saudi J. Biol. Sci. 2019, 26, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Boutkhil, S.; El Idrissi, M.; Chakir, S.; Derraz, M.; Amechrouq, A.; Chbicheb, A.; El Badaoui, K. Antibacterial and antifungal activity of extracts and essential oils of Seriphidium herba-alba (Asso) Soják and their combination effects with the essential oils of Dysphania ambrosioides (L) Mosyakin & Clemants. Acta Bot. Gall. 2011, 158, 425–433. [Google Scholar]
- Standard, A. NCCLS Document M27-A; NCCLS: Wayne, PA, USA, 1997. [Google Scholar]


| Chemical Name | Retention Index (RI) | Percentages (%) | |||||
|---|---|---|---|---|---|---|---|
| Pinus halepensis | Pinus pinea | ||||||
| AI | RI | Ph-1 | Ph-2 | Pp-1 | Pp-2 | ||
| Monoterpene | |||||||
| 1. | α-Pinene | 932 | 939 | 8.54 ± 0.50 a | 13.33 ± 0.21 b | 13.82 ± 0.03 b | 20.97 ± 0.16 c |
| 2. | Sabinene | 970 | 976 | 3.15 ± 0.20 | − | − | − |
| 3. | β-pinene | 974 | 980 | 4.08 ± 0.31 | − | − | − |
| 4. | β-Myrcene | 990 | 990 | 5.80 ± 0.05 a | − | − | 1.37 ± 0.06 b |
| 5. | δ-3-carene | 1008 | 1011 | 4.07 ± 0.19 | − | − | − |
| 6. | p-Cymene | 1022 | 1022 | 1.39 ± 1.10 a | - | 0.82 ± 0.21 a | 0.69 ± 0.11 a |
| 7. | Limonene | 1024 | 1029 | 1.49 ± 0.02 a | 6.77 ± 0.02 b | 1.13 ± 0.14 a | 8.49 ± 0.01 b |
| 8. | γ-terpinene | 1054 | 1055 | 3.38 ± 0.19 a | − | 3.18 ± 0.09 a | − |
| 9. | α-Terpinolene | 1018 | 1088 | 1.08 ± 0.05 a | − | 5.67 ± 0.12 b | 4.75 ± 0.21 b |
| 27.18 | 20.1 | 24.62 | 36.27 | ||||
| Oxygenated monoterpenes | |||||||
| 10. | α-Campholenal | 1130 | 1128 | − | − | 1.85 ± 0.02 a | 0.63 ± 0.02 a |
| 11. | trans-pinocarveol | 1135 | 1136 | − | − | 4.89 ± 0.25 a | 1.08 ± 0.12 b |
| 12. | Pinocarvone | 1160 | 1164 | − | − | 1.77 ± 0.31 a | 1.35 ± 0.05 a |
| 13. | Borneol | 1165 | 1168 | − | − | 2.47 ± 0.42 a | 0.91 ± 0.14 a |
| 14. | Myrtenol | 1194 | 1195 | − | − | 1.20 ± 0.29 | - |
| 15. | Verbenone | 1204 | 1206 | − | − | 0.56 ± 0.00 | - |
| 16. | trans-(+)-carveol | 1215 | 1219 | − | − | 1.04 ± 0.17 | - |
| 17. | Carveol methyl ether | 1229 | 1229 | − | − | 1.09 ± 0.07 | - |
| 18. | Carvone | 1239 | 1236 | − | − | 0.57 ± 0.12 | - |
| 19. | Bornyl acetate | 1287 | 1285 | − | − | 1.49 ± 0.06 a | 1.29 ± 0.07 a |
| 20. | Thymol | 1232 | 1232 | − | − | 5.53 ± 0.05 a | 0.65 ± 0.01 b |
| 21. | Carvacrol | 1298 | 1298 | − | − | 0.90 ± 0.23 a | 2.08 ± 0.00 a |
| Zero | Zero | 23.36 | 7.99 | ||||
| Sesquiterpene hydrocarbons | |||||||
| 22. | α-Cubebene | 1345 | 1348 | − | − | 1.42 ± 0.09 a | 2.64 ± 0.02 a |
| 23. | α-longipinene | 1350 | 1350 | 3.47 ± 0.19 a | − | 2.80 ± 0.01 a | 2.64 ± 0.19 a |
| 24. | β-Elemene | 1389 | 1390 | − | 2.06 ± 0.07 a | 1.26 ± 0.05 a | − |
| 25. | β-Caryophyllene | 1417 | 1417 | 5.29 ± 0.00 a | 6.20 ± 0.19 a | 1.65 ± 0.12 b | 5.39 ± 0.01 a |
| 26. | Aromadenderene | 1439 | 1430 | 1.61 ± 0.12 a | 1.38 ± 0.12 a | 1.01 ± 0.42 a | − |
| 27. | α-Humulene | 1452 | 1454 | 3.12 ± 0.05 a | 2.0 ± 0.29 a | 1.19 ± 0.41 b | 3.39 ± 0.20 a |
| 28. | Germacrene D | 1484 | 1485 | − | 0.68 ± 0.02 a | − | 7.81 ± 0.03 b |
| 29. | δ-amorphene | 1511 | 1507 | − | − | 1.82 ± 0.57 a | 4.54 ± 0.11 b |
| 30. | Cis-α-bisabolene | 1508 | 1508 | − | − | 1.38 ± 0.67 a | 0.65 ± 0.09 a |
| 31. | γ-cadinene | 1514 | 1514 | − | − | 0.21 ± 0.03 | − |
| 32. | δ-cadinene | 1523 | 1522 | 2.63 ± 0.01 a | 2.03 ± 0.09 a | 4.54 ± 0.07 b | 2.62 ± 0.02 a |
| 16.12 | 14.35 | 19.95 | 33.16 | ||||
| Oxygenated sesquiterpenes | |||||||
| 33. | Caryophyllene oxide | 1582 | 1581 | 1.39 ± 0.03 a | 4.45 ± 0.03 b | 1.70 ± 0.54 a | 3.55 ± 0.07 b |
| 34. | Aromadendrene oxide | 1595 | 1595 | 2.37 ± 0.10 a | 12.13 ± 0.36 b | 3.63 ± 0.21 a | 1.57 ± 0.08 a |
| 35. | Cubenol | 1618 | 1616 | − | − | 0.89 ± 0.15 a | 0.92 ± 0.15 a |
| 36. | Muurolol | 1640 | 1642 | − | − | 2.59 ± 0.00 a | 2.95 ± 0.09 a |
| 37. | Vulgarol B | 1688 | 1688 | − | − | 2.55 ± 0.02 a | 1.74 ± 0.01 a |
| 3.76 | 16.58 | 11.36 | 10.73 | ||||
| Diterpene hydrocarcons | |||||||
| 38. | Cembrene | 1937 | 1932 | 2.54 ± 0.05 a | 8.23 ± 0.07 b | 2.10 ± 0.02 a | − |
| 2.54 | 8.23 | 2.1 | − | ||||
| Oxygenated diterpenes | |||||||
| 39. | Manool oxide | 1987 | 1994 | 3.94 ± 0.02 a | 2.55 ± 0.25 b | 5.06 ± 0.00 a | 2.25 ± 0.15 b |
| 40. | Thunbergol | 2047 | 2047 | 5.76 ± 0.58 a | 18.85 ± 0.17 b | 6.16 ± 0.02 a | − |
| 41. | 1,4-menthano-azulene | 2110 | 2110 | − | − | 2.67 ± 0.09 a | 3.48 ± 0.07 a |
| 42. | Dehydro abietinal | 2279 | 2279 | 4.14 ± 0.10 | − | − | − |
| 43. | Kaur-16-en-19-ol | 2346 | 2346 | 3.28 ± 0.21 | − | − | − |
| 44. | Methyl dehydroabietate | 2359 | 2354 | 7.78 ± 0.03 a | − | 0.71 ± 0.19 b | − |
| 45. | Dehydro abietic acid | 2380 | 2380 | 5.74 ± 0.31 a | 1.38 ± 0.27 b | 0.71 ± 0.30 b | − |
| 46. | Abietic acid methyl ester | 2377 | 2380 | 3.35 ± 0.06 a | − | 0.84 ± 0.09 b | − |
| 33.99 | 22.78 | 12.64 | 2.25 | ||||
| Miscellaneous compounds | |||||||
| 47. | 2-pentadecanone | 1694 | 1698 | − | − | 1.59 ± 0.01 a | 0.70 ± 0.21 a |
| 48. | Pentadecanal | 1716 | 1716 | − | − | 1.91 ± 0.09 | − |
| − | − | 4.34 | 0.7 | ||||
| Total % | 89.39 ± 4.10 | 82.04 ± 2.16 | 94.03 ± 6.75 | 91.1 ± 2.46 | |||
| Yield% (v/w) | 0.22 | 0.59 | 0.12 | 0.22 | |||
| Inhibition Zones | Diameter of Inhibition in mm | ||||||
|---|---|---|---|---|---|---|---|
| Micro- Organisms | P. halepensis | P. pinea | Cefotaxime | Nystatin | |||
| Pp-2 | Pp-1 | Ph-2 | Ph-1 | ||||
| Gram-Positive | |||||||
| Bacillus subtilis | 8 ± 0.32 | 18 ± 0.91 | − | − | 10 ± 0.89 | ||
| Micrococcus lutea | 39 ± 0.54 | 39 ± 0.83 | − | − | 27 ± 0.93 | ||
| Gram-Negative | |||||||
| Escherichia coli | 10 ± 0.51 | 18 ± 0.90 | 18 ± 0.84 | 8 ± 0.36 | 27 ± 0.13 | ||
| Proteus mirabilis | 14 ± 0.56 | 15 ± 0.71 | − | − | 25 ± 0.83 | ||
| Fungi | |||||||
| Candida albican | 10 ± 0.25 | 15 ± 0.13 | − | − | 18 ± 0.34 | ||
| Gram Negative | Gram Positive | Fungi | |||
|---|---|---|---|---|---|
| Bacillus subtilis | Micrococcus Lutea | E. coli | Proteus mirabilis | Candida albicans | |
| Cefotaxime | 10 | 27 | 27 | 25 | − |
| Erythromycin | 26 | 14 | − | − | − |
| Polymyxin-B | 11 | 18 | 12 | − | − |
| Cephalexin | 10 | 21 | − | − | − |
| Amoxicillin | − | − | − | 15 | − |
| Tetracycline | 27 | 29 | 21 | − | − |
| Streptomycin | 20 | 27 | − | 15 | − |
| Nalidixic Acid | 20 | 17 | − | 20 | − |
| Fusidic Acid | 23 | 20 | − | - | − |
| Amoxyclav | 11 | 14 | − | 25 | − |
| Carbenicillin | 9 | 11 | − | 28 | − |
| Gentamicin | 22 | 28 | 19 | 20 | − |
| Chloramphenicol | 15 | 23 | 23 | − | − |
| Amikacin | 27 | 21 | 19 | 21 | − |
| Ceftriaxone | 12 | 20 | 27 | 20 | − |
| Nystatin | 18 | ||||
| Localities | Altitude (m) | Geographical Coordinates | ||
|---|---|---|---|---|
| P. halepensis (Ph-1) | Sidi Alhamry | 830 m | 32°38′286″ N | 21°48′164″ E |
| P. halepensis (Ph-2) | Alaslab | 75 m | 32°54′458″ N | 22°09′587″ E |
| P. pinea (Pp-1) | Werdama | 625 m | 32°47′334″ N | 21°46′313″ E |
| P. pinea (Pp-2) | Al-Mansura | 408 m | 32°50′10″ N | 21°51′10″ E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkady, W.M.; Gonaid, M.H.; Yousif, M.F.; El-Sayed, M.; Omar, H.A.N. Impact of Altitudinal Variation on the Phytochemical Profile, Anthelmintic and Antimicrobial Activity of Two Pinus Species. Molecules 2021, 26, 3170. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113170
Elkady WM, Gonaid MH, Yousif MF, El-Sayed M, Omar HAN. Impact of Altitudinal Variation on the Phytochemical Profile, Anthelmintic and Antimicrobial Activity of Two Pinus Species. Molecules. 2021; 26(11):3170. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113170
Chicago/Turabian StyleElkady, Wafaa M., Mariam H. Gonaid, Miriam F. Yousif, Mahmoud El-Sayed, and Hind A. N. Omar. 2021. "Impact of Altitudinal Variation on the Phytochemical Profile, Anthelmintic and Antimicrobial Activity of Two Pinus Species" Molecules 26, no. 11: 3170. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113170