Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples
Abstract
:1. Introduction
2. Results and Discussion
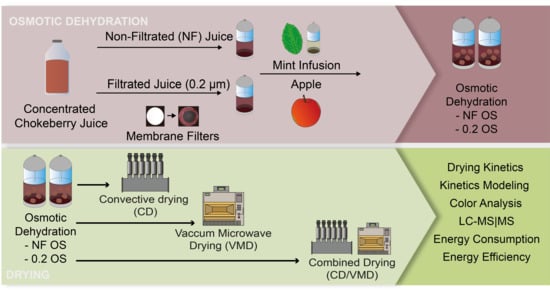
2.1. Osmotic Dehydration
2.2. Drying Kinetics
2.3. Color
2.4. Phenolic Compounds
2.5. Energy Consumption during Drying
3. Materials and Methods
3.1. Materials
3.2. Osmotic Dehydration
3.3. Drying Methods
3.4. Concentration of Osmotic Solutions
3.5. Moisture Content
3.6. Color
3.7. Energy Consumption
3.8. Specific Energy Consumption
3.9. Energy Efficiency
3.10. Energy Savings
3.11. Chemical Analysis
3.11.1. Sample Preparation
3.11.2. LC-MS/MS Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Almeida, J.A.R.; Mussi, L.P.; Oliveira, D.B.; Pereira, N.R. Effect of Temperature and Sucrose Concentration on the Retention of Polyphenol Compounds and Antioxidant Activity of Osmotically Dehydrated Bananas: Bioactive Compounds in the Osmotic Dehydration. J. Food Process. Preserv. 2015, 39, 1061–1069. [Google Scholar] [CrossRef]
- Mrad, N.D.; Bonazzi, C.; Courtois, F.; Kechaou, N.; Mihoubi, N.B. Moisture Desorption Isotherms and Glass Transition Temperatures of Osmo-Dehydrated Apple and Pear. Food Bioprod. Process. 2013, 91, 121–128. [Google Scholar] [CrossRef]
- Lech, K.; Michalska, A.; Wojdyło, A.; Nowicka, P.; Figiel, A. The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution. Molecules 2017, 22, 2246. [Google Scholar] [CrossRef] [Green Version]
- Bchir, B.; Besbes, S.; Karoui, R.; Paquot, M.; Attia, H.; Blecker, C. Osmotic Dehydration Kinetics of Pomegranate Seeds Using Date Juice as an Immersion Solution Base. Food Bioprocess Technol. 2012, 5, 999–1009. [Google Scholar] [CrossRef]
- Masztalerz, K.; Łyczko, J.; Lech, K.; Szumny, A.; Figiel, A. The Effect of Filtrated Osmotic Solutions Based on Chokeberry Juice Enriched with Mint Extract on Volatile Compounds in Dried Apples. J. Food Process Eng. 2021. [Google Scholar] [CrossRef]
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzyńska, A.; Lenart, A.; Kowalska, H. The Use of Antioxidant Potential of Chokeberry Juice in Creating Pro-Healthy Dried Apples by Hybrid (Convection-Microwave-Vacuum) Method. Molecules 2020, 25, 5680. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Eliasson, L.; Marzec, A.; Kowalska, J.; Piotrowski, D.; Lenart, A.; Kowalska, H. The Effect of Adding Berry Fruit Juice Concentrates and By-Product Extract to Sugar Solution on Osmotic Dehydration and Sensory Properties of Apples. J. Food Sci. Technol. 2019, 56, 1927–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, H.; Marzec, A.; Domian, E.; Masiarz, E.; Ciurzyńska, A.; Galus, S.; Małkiewicz, A.; Lenart, A.; Kowalska, J. Physical and Sensory Properties of Japanese Quince Chips Obtained by Osmotic Dehydration in Fruit Juice Concentrates and Hybrid Drying. Molecules 2020, 25, 5504. [Google Scholar] [CrossRef]
- Pavez-Guajardo, C.; Ferreira, S.R.S.; Mazzutti, S.; Guerra-Valle, M.E.; Sáez-Trautmann, G.; Moreno, J. Influence of In Vitro Digestion on Antioxidant Activity of Enriched Apple Snacks with Grape Juice. Foods 2020, 9, 1681. [Google Scholar] [CrossRef]
- Phisut, N. Factors Affecting Mass Transfer during Osmotic Dehydration of Fruits. Int. Food Res. J. 2012, 19, 7–18. [Google Scholar]
- Saurel, R.; Raoult-Wack, A.-L.; Rios, G.; Guilbert, S. Mass Transfer Phenomena during Osmotic Dehydration of Apple I. Fresh Plant Tissue. Int. J. Food Sci. Technol. 1994, 29, 531–542. [Google Scholar] [CrossRef]
- Goula, A.M.; Kokolaki, M.; Daftsiou, E. Use of Ultrasound for Osmotic Dehydration. The Case of Potatoes. Food Bioprod. Process. 2017, 105, 157–170. [Google Scholar] [CrossRef]
- Antonyuk, S.; Heinrich, S.; Deen, N.; Kuipers, H. Influence of Liquid Layers on Energy Absorption during Particle Impact. Particuology 2009, 7, 245–259. [Google Scholar] [CrossRef]
- Lech, K.; Michalska, A.; Wojdyło, A.; Nowicka, P.; Figiel, A. The Influence of Physical Properties of Selected Plant Materials on the Process of Osmotic Dehydration. LWT Food Sci. Technol. 2018, 91, 588–594. [Google Scholar] [CrossRef]
- Masztalerz, K.; Figiel, A.; Michalska-Ciechanowska, A.; Wojdyło, A.; Nowicka, P.; Lech, K. The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material. Molecules 2020, 25, 5412. [Google Scholar] [CrossRef] [PubMed]
- Çağlayan, D.; Barutçu Mazı, I. Effects of Ultrasound-Assisted Osmotic Dehydration as a Pretreatment and Finish Drying Methods on the Quality of Pumpkin Slices. J. Food Process. Preserv. 2018, 42, e13679. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lech, K.; Michalska, A.; Wasilewska, M.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Influence of Osmotic Dehydration Pre-Treatment and Combined Drying Method on Physico-Chemical and Sensory Properties of Pomegranate Arils, Cultivar Mollar de Elche. Food Chem. 2017, 232, 306–315. [Google Scholar] [CrossRef]
- Castillo-Gironés, S.; Masztalerz, K.; Lech, K.; Issa-Issa, H.; Figiel, A.; Carbonell-Barrachina, Á.A. Impact of Osmotic Dehydration and Different Drying Methods on the Texture and Sensory Characteristic of Sweet Corn Kernels. J. Food Process. Preserv. 2021, 45, e15383. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Chemical Determinants of Dried Thai Basil (O. basilicum Var. thyrsiflora) Aroma Quality. Ind. Crops Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The Effect of Drying Methods on the Energy Consumption, Bioactive Potential and Colour of Dried Leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef] [Green Version]
- Calín-Sánchez, Á.; Lech, K.; Szumny, A.; Figiel, A.; Carbonell-Barrachina, Á.A. Volatile Composition of Sweet Basil Essential Oil (Ocimum basilicum L.) as Affected by Drying Method. Food Res. Int. 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Ghasemi Pirbalouti, A. Quantity and Chemical Composition of Essential Oil of Peppermint (Mentha × piperita L.) Leaves under Different Drying Methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Joardder, M.U.H.; Alsbua, R.; Akram, W.; Karim, M.A. Effect of Sample Rugged Surface on Energy Consumption and Quality of Plant-Based Food Materials in Convective Drying. Dry. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Serwatka, Z. Influence of Blanching and Convective Drying Conditions of Parsley on Process Energy Consumption. Teka Kom. Motoryz. Energetyki Rol. 2011, 11C, 142–150. [Google Scholar]
- Calín-Sanchez, Á.; Figiel, A.; Szarycz, M.; Lech, K.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A. Drying Kinetics and Energy Consumption in the Dehydration of Pomegranate (Punica granatum L.) Arils and Rind. Food Bioprocess Technol. 2014, 7, 2071–2083. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Wojdyło, A.; Szarycz, M.; Carbonell-Barrachina, Á.A. Drying of Garlic Slices Using Convective Pre-Drying and Vacuum-Microwave Finishing Drying: Kinetics, Energy Consumption, and Quality Studies. Food Bioprocess Technol. 2014, 7, 398–408. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á.A. Effects of Drying Methods on the Composition of Thyme (Thymus vulgaris L.) Essential Oil. Dry. Technol. 2013, 31, 224–235. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; ISBN 978-0-19-853344-3. [Google Scholar]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia Alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef] [Green Version]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; Masztalerz, K.; Szumny, A. HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules 2019, 24, 764. [Google Scholar] [CrossRef] [Green Version]
- Nöfer, J.; Lech, K.; Figiel, A.; Szumny, A.; Carbonell-Barrachina, Á.A. The Influence of Drying Method on Volatile Composition and Sensory Profile of Boletus edulis. J. Food Qual. 2018, 2018, 2158482. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Influence of Osmodehydration Pretreatment and Combined Drying Method on the Bioactive Potential of Sour Cherry Fruits. Food Bioprocess Technol. 2015, 8, 824–836. [Google Scholar] [CrossRef] [Green Version]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Michalska, A.; Wojdyło, A.; Nowicka, P. The Effect of Selected Fruit Juice Concentrates Used as Osmotic Agents on the Drying Kinetics and Chemical Properties of Vacuum-Microwave Drying of Pumpkin. J. Food Qual. 2018, 2018, 7293932. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M.; Mengeş, H.O. Evaluation of Drying Methods with Respect to Drying Parameters, Some Nutritional and Colour Characteristics of Peppermint (Mentha × piperita L.). Energy Convers. Manag. 2010, 51, 2769–2775. [Google Scholar] [CrossRef]
- Doymaz, İ. Infrared Drying of Sweet Potato (Ipomoea batatas L.) Slices. J. Food Sci. Technol. 2012, 49, 760–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doymaz, İ. Thin-Layer Drying Behaviour of Mint Leaves. J. Food Eng. 2006, 74, 370–375. [Google Scholar] [CrossRef]
- Lenart, A.; Lewicki, P. Osmotic Dehydration of Fruits and Vegetables. In Handbook of Industrial Drying, 3rd ed.; Mujumdar, A., Ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1-57444-668-5. [Google Scholar]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of Sonication on Colour, Ascorbic Acid and Yeast Inactivation in Tomato Juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of Absorbance Values Used in Wineries for Estimating CIELAB Parameters in Red Wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.; Nieman, K.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [Green Version]
- Lachowicz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Stokłosa, D. Effect of Different Sizes of Ceramic Membranes in the Process of Microfiltration on Physicochemical Parameters of Chokeberry Juice. Eur. Food Res. Technol. 2019, 245, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- Sarsavadia, P.N. Development of a Solar-Assisted Dryer and Evaluation of Energy Requirement for the Drying of Onion. Renew. Energy 2007, 32, 2529–2547. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, M.A. Experimental Investigation of Bound and Free Water Transport Process during Drying of Hygroscopic Food Material. Int. J. Therm. Sci. 2017, 117, 266–273. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Zambra, C.; Hernández, D.; Reyes, H.; Riveros, N.; Lemus-Mondaca, R. Kageneckia Oblonga Leaves Subjected to Different Drying Methods: Drying Kinetics, Energy Consumption and Interesting Compounds. Front. Sustain. Food Syst. 2021, 5, 641858. [Google Scholar] [CrossRef]
- Amado, L.R.; de Souza Silva, K.; Mauro, M.A. Drying of Mangoes (Mangifera indica L. cv. Palmer) at Changeable Temperature Conditions—Effects on Energy Consumption and Quality of the Dehydrated Fruit. J. Food Process Eng. 2021, 44, e13615. [Google Scholar] [CrossRef]
- Masztalerz, K.; Lech, K.; Wojdyło, A.; Nowicka, P.; Michalska-Ciechanowska, A.; Figiel, A. The Impact of the Osmotic Dehydration Process and Its Parameters on the Mass Transfer and Quality of Dried Apples. Dry. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Dandamrongrak, R.; Young, G.; Mason, R. Evaluation of Various Pre-Treatments for the Dehydration of Banana and Selection of Suitable Drying Models. J. Food Eng. 2002, 55, 139–146. [Google Scholar] [CrossRef]
- Hendorson, S.M. Grain Drying Theory (I) Temperature Effect on Drying Coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Michalska, A.; Wojdyło, A.; Łysiak, G.; Figiel, A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant Property and Storage Stability of Quince Juice Phenolic Compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef]





| SG, g·g−1 fw | WL, g·g−1 fw | WL/SG | EODw, kJ·g−1 w | EODm, kJ·g−1 fw | |
|---|---|---|---|---|---|
| 0.2 OS | 0.050 ± 0.006 a,† | 0.218 ± 0.008 a | 4.228 ± 0.27 a | 578.67 ± 22.88 a | 3.54 ± 0.02 a |
| NF OS | 0.050 ± 0.002 a | 0.212 ± 0.021 a | 4.216 ± 0.34 a | 597.86 ± 60.54 a | 3.57 ± 0.02 a |
| Model | Drying Conditions | Constants | Statistics | |||
|---|---|---|---|---|---|---|
| A | k | B | RMSE | R2 | ||
| Modified Page | 0.2/CD | 0.525 | 0.0157 | 1.12 | 0.0091 | 0.9972 |
| NF/CD | 0.513 | 0.0118 | 1.19 | 0.0080 | 0.9978 | |
| 0.2/CD/VMD | 0.122 | 0.5620 | 0.91 | 0.0051 | 0.9806 | |
| NF/CD/VMD | 0.141 | 0.4280 | 1.22 | 0.0054 | 0.9844 | |
| 0.2/VMD | 0.527 | 0.1220 | 1.37 | 0.0094 | 0.9969 | |
| NF/VMD | 0.540 | 0.1070 | 1.43 | 0.0084 | 0.9972 | |
| A | k | C | RMSE | R2 | ||
| Logarithmic | 0.2/CD | 0.538 | 0.0263 | 0.0047 | 0.0107 | 0.9962 |
| NF/CD | 0.536 | 0.0261 | 0.0023 | 0.0126 | 0.9946 | |
| 0.2/CD/VMD | 0.117 | 0.5790 | 0.0052 | 0.0013 | 0.9987 | |
| NF/CD/VMD | 0.137 | 0.5720 | 0.0041 | 0.0042 | 0.9906 | |
| 0.2/VMD | 0.556 | 0.2280 | −0.0064 | 0.0231 | 0.9812 | |
| NF/VMD | 0.576 | 0.2340 | −0.0083 | 0.0262 | 0.9777 | |
| A | k | RMSE | R2 | |||
| Henderson–Pabis | 0.2/CD | 0.541 | 0.0257 | 0.0109 | 0.9960 | |
| NF/CD | 0.538 | 0.0258 | 0.0124 | 0.9949 | ||
| 0.2/CD/VMD | 0.121 | 0.5120 | 0.0047 | 0.984 | ||
| NF/CD/VMD | 0.141 | 0.5280 | 0.0052 | 0.9857 | ||
| 0.2/VMD | 0.551 | 0.2350 | 0.0223 | 0.9829 | ||
| NF/VMD | 0.569 | 0.2320 | 0.0253 | 0.9794 | ||
| Method | L* | a* | b* | ∆E* | TM, °C | t, min | tR, min | t5%wb, min | ES, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VMD | CD | CD | VMD | ||||||||
| Fresh | 71.16 ± 3.21 | −2.46 ± 0.45 | 10.62 ± 1.25 | - | - | - | - | - | - | - | - |
| 0.2/CD | 25.49 ± 2.07 b,† | 14.59 ± 5.24 a,b | 1.51 ± 1.45 a | 49.6 ± 0.37 a | 60 | 360 | - | - | 285 | - | - |
| NF/CD | 24.1 ± 0.51 a,b | 8.58 ± 2.64 a | 0.22 ± 0.34 a | 49.45 ± 0.88 a | 60 | 360 | - | - | 288 | - | - |
| 0.2/CD/VMD | 22.21 ± 0.25 a | 13.63 ± 2.76 a,b | 1.55 ± 0.83 a | 52.32 ± 0.85 b | 71 | 60 | 38 | 6 | 60 | 6 ± 1 | 53.2 ± 0.5 a |
| NF/CD/VMD | 23.39 ± 1.53 a,b | 16.86 ± 5.37 b | 2.12 ± 1.26 a | 52.22 ± 1.01 b | 70 | 60 | 38 | 6 | 60 | 6 ± 1 | 57 ± 0.4 b |
| 0.2/VMD | 21.55 ± 1.13 a | 13.24 ± 2.53 a,b | 0.68 ± 0.64 a | 52.98 ± 0.77 b | 81 | - | 44 | 12 | - | 12 ± 1 | 80.4 ± 0.2 c |
| NF/VMD | 23.87 ± 1.52 a,b | 15.62 ± 3.08 a,b | 0.71 ± 0.91 a | 51.59 ± 0.65 b | 80 | - | 44 | 12 | - | 13 ± 1 | 80.2 ± 0.2 c |
| Apple | |||
| Samples | CA mg per 100 g | RA mg per 100 g | ChA mg per 100 g |
| CD NF | 10.873 ± 0.970 b,c,† | 0.274 ± 0.065 a | 260.254 ± 9.391 a |
| CD 0.2 | 11.205 ± 0.226 c | 0.193 ± 0.021 a | 236.512 ± 12.804 a,b |
| CD/VMD NF | 9.455 ± 0.138 a | 0.415 ± 0.034 b | 223.99 ± 7.238 b |
| CD/VMD 0.2 | 9.592 ± 0.135 a | 0.438 ± 0.041 b | 222.477 ± 11.59 b |
| VMD NF | 9.864 ± 0.137 a,b | 0.422 ± 0.019 b | 219.658 ± 8.810 b |
| VMD 0.2 | 10.505 ± 0.446 a,b,c | 0.432 ± 0.021 b | 225.255 ± 6.222 b |
| Osmotic Solution | |||
| Samples | CA mg per 100 mL | RA mg per 100 mL | ChA mg per 100 mL |
| NF + M 0’ | 4.382 ± 0.230 b | 9.847 ± 0.034 a | 179.076 ± 9.401 a |
| NF + M 30’ | 2.509 ± 0.405 a | 7.333 ± 0.577 b | 131.140 ± 12.774 c |
| 0.2 + M 0’ | 4.713 ± 0.441 b | 4.434 ± 0.277 c | 162.352 ± 8.404 a,b |
| 0.2 + M 30’ | 3.998 ± 0.668 b | 3.331 ± 0.278 d | 148.615 ± 9.870 b,c |
| Model | Model Equation | Reference |
|---|---|---|
| Modified Page | [26] | |
| Logarithmic | [49] | |
| Henderson–Pabis | [50] |
| Compound | Precursor m/z [M − H]− | MRM Transitions m/z (Q1 → Q3) | Q1 Pre Bias(V) | Collision Energy | Q3 Pre Vias(V) |
|---|---|---|---|---|---|
| Caffeic acid | 178.8000 | 178.8000→135.3000 178.8000→134.2500 178.8000→79.25000 | 20.0 20.0 20.0 | 18.0 26.0 24.0 | 26.0 25.0 14.0 |
| Chlorogenic acid | 353.0000 | 353.0000→191.3000 353.0000→85.2000 353.0000→93.2500 | 18.0 18.0 17.0 | 15.0 42.0 45.0 | 12.0 17.0 18.0 |
| Rosmarinic acid | 359.1000 | 359.1000→161.3000 359.1000→197.3000 359.1000→133.2500 | 18.0 18.0 18.0 | 16.0 17.0 40.0 | 20.0 12.0 26.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masztalerz, K.; Łyczko, J.; Lech, K. Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples. Molecules 2021, 26, 3274. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113274
Masztalerz K, Łyczko J, Lech K. Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples. Molecules. 2021; 26(11):3274. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113274
Chicago/Turabian StyleMasztalerz, Klaudia, Jacek Łyczko, and Krzysztof Lech. 2021. "Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples" Molecules 26, no. 11: 3274. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113274