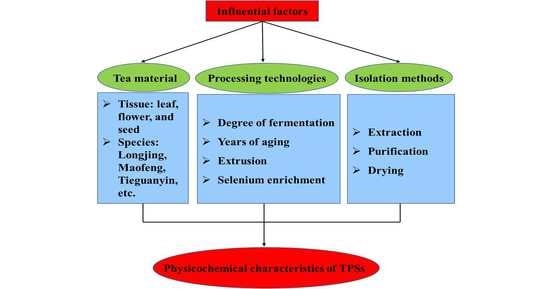
Influencing Factors on the Physicochemical Characteristics of Tea Polysaccharides
Abstract
:1. Introduction
2. Preparation of TPSs
3. Physicochemical Characterization of TPSs
3.1. Tea Material
3.2. Processing Technologies
3.3. Isolation Methods
4. Applications of TPSs
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| Ara | Arabinose |
| BWE | Boiling water extraction |
| EE | Enzyme extraction |
| FT-IR | Fourier transform infrared spectroscopy |
| Fuc | Fucose |
| Glc | Glucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GluA | Glucuronic acid |
| GC | Gas chromatography |
| GC-MS | GC-mass spectroscopy |
| GFC | Gel-filtration chromatography |
| GPC | Gel permeation chromatography |
| HG | Homogalacturonan |
| HWE | Hot water extraction |
| Man | Mannose |
| MAE | Microwave-assisted extraction |
| MLLS | Multiangle laser light-scattering instrument |
| Mw | Molecular weight |
| MWD | Molecular weight distribution |
| NMR | Nuclear magnetic resonance spectroscopy |
| Rha | Rhamnose |
| Rib | Ribose |
| TFA | Trifluoroacetic acid |
| TPC | Tea polysaccharide conjugates |
| TPS | Tea polysaccharides |
| TWE | Traditional water extraction |
| TEM | Transmission electron microscopy |
| UAE | Ultrasound-assisted extraction |
| Xyl | Xylose |
References
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.B.; Jiang, H. A review on the structure-function relationship aspect of polysaccharides from tea materials. Crit. Rev. Food Sci. Nutr. 2015, 55, 930–938. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Y.; Zhang, Q.; Zhang, K.; Peng, P.; Chen, L.; Xiao, B. Hydrothermal extraction, structural characterization, and inhibition HeLa cells proliferation of functional polysaccharides from Chinese tea Zhongcha 108. J. Funct. Foods 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Nie, S.-P.; Xie, M.-Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Nie, S.; Cui, S.W.; Xie, M. Chapter 7—Tea polysaccharide. In Bioactive Polysaccharides; Nie, S., Cui, S.W., Xie, M., Eds.; Academic Press: London, UK, 2018; pp. 349–394. [Google Scholar]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Yu, Q.Y.; Jiang, S.; Xiong, C.Y.; Ling, Z.J.; He, P.M. Structural characterization and antioxidant activities of 2 water-soluble polysaccharide fractions purified from tea (Camellia sinensis) flower. J. Food Sci. 2011, 76, C462–C471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, F.; Wei, X. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Xiao, J.; Huo, J.; Jiang, H.; Yang, F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011, 49, 1143–1151. [Google Scholar] [CrossRef]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.-W.; Gao, Z.-Y.; Xiao, W.; Li, T.-Q.; Song, W.; Zheng, J.; Chen, H.; Chen, G.-H.; Zou, H.-Y. MiR-93 functions as a tumor promoter in prostate cancer by targeting disabled homolog 2 (DAB2) and an antitumor polysaccharide from green tea (Camellia sinensis) on their expression. Int. J. Biol. Macromol. 2019, 125, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Gao, Z.-Y.; Li, T.-Q.; Song, W.; Xiao, W.; Zheng, J.; Chen, H.; Chen, G.-H.; Zou, H.-Y. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 2019, 122, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Z.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.; Hu, X.; Wei, X.; Wang, Y. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef]
- Wang, H.; Shi, S.; Bao, B.; Li, X.; Wang, S. Structure characterization of an arabinogalactan from green tea and its anti-diabetic effect. Carbohydr. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef]
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.D.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Yin, L.; Fu, S.; Wu, R.; Wei, S.; Yi, J.; Zhang, L.-M.; Yang, L. A neutral polysaccharide from green tea: Structure, effect on α-amylase activity and hydrolysis property. Arch. Biochem. Biophys. 2020, 687, 108369. [Google Scholar] [CrossRef]
- Yin, L.; Fu, S.; Wu, R.; Wei, S.; Yi, J.; Zhang, L.-M.; Yang, L. Chain conformation of an acidic polysaccharide from green tea and related mechanism of α-amylase inhibitory activity. Int. J. Biol. Macromol. 2020, 164, 1124–1132. [Google Scholar] [CrossRef]
- Wang, H.; Wei, G.; Liu, F.; Banerjee, G.; Joshi, M.; Bligh, S.W.A.; Shi, S.; Lian, H.; Fan, H.; Gu, X.; et al. Characterization of two homogalacturonan pectins with immunomodulatory activity from green tea. Int. J. Mol. Sci. 2014, 15, 9963–9978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Bai, Y.; Zeng, Z.; Peng, Y.; Zhou, W.; Shen, W.; Zeng, X.; Liu, Z. Structural characterization and immunostimulatory activity of heteropolysaccharides from Fuzhuan Brick tea. J. Agric. Food. Chem. 2021, 69, 1368–1378. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, L.Y. Supercritical extraction technology in tea polysaccharide extracting application. Adv. Mater. Res. 2012, 347, 1683–1688. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Guo, Y.; Xiao, J.; Wang, Y. Composition and biological activity of tea polysaccharides obtained by water extraction and enzymatic extraction. Lat. Am. J. Pharm. 2010, 29, 117–121. [Google Scholar]
- Wang, J.; Liu, W.; Chen, Z.; Chen, H. Physicochemical characterization of the oolong tea polysaccharides with high molecular weight and their synergistic effects in combination with polyphenols on hepatocellular carcinoma. Biomed. Pharmacother. 2017, 90, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Z.; Chen, L.; Yu, C.; Wang, H.; Wei, X.; Wang, Y. Comparison and structural characterization of polysaccharides from natural and artificial Se-enriched green tea. Int. J. Biol. Macromol. 2019, 130, 388–398. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Jin, Z. Structure analysis of an acidic polysaccharide isolated from green tea. Nat. Prod. Res. 2009, 23, 678–687. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of selenium-containing polysaccharides isolated from selenium-enriched tea and its bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef]
- Peng, Z.; Xie, M.; Nie, S.; Wang, X. Primary structure and configuration of tea polysaccharide. Sci. China Ser. C Life Sci. 2004, 47, 416–424. [Google Scholar]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, S.; Xie, J.; Yuan, H.; Li, Q.; Wu, L.; Wu, Z.; Yuan, H.; Jiang, Y. Analysis of protein moiety of polysaccharide conjugates water-extracted from low grade green tea. Chem. Res. Chin. Univ. 2018, 34, 691–696. [Google Scholar] [CrossRef]
- Wu, S.; Lai, M.; Luo, J.; Pan, J.; Zhang, L.-M.; Yang, L. Interactions between α-amylase and an acidic branched polysaccharide from green tea. Int. J. Biol. Macromol. 2017, 94, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Scoparo, C.T.; de Souza, L.M.; Rattmann, Y.D.; Dartora, N.; Paiva, S.M.M.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Polysaccharides from green and black teas and their protective effect against murine sepsis. Food Res. Int. 2013, 53, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhao, Y.; Sun, Y.; Yang, S.; Yang, X. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Gu, Y. Pharmacological evaluation of tea polysaccharides with antioxidant activity in gastric cancer mice. Carbohydr. Polym. 2012, 90, 943–947. [Google Scholar] [CrossRef]
- Yang, L.; Fu, S.; Zhu, X.; Zhang, L.-M.; Yang, Y.; Yang, X.; Liu, H. Hyperbranched acidic polysaccharide from green tea. Biomacromolecules 2010, 11, 3395–3405. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.; Ye, Y.; Zhang, R.; Yin, J.; Jiang, Y.; Wan, H. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of water-soluble and alkali-soluble polysaccharide conjugates prepared from green tea. Carbohydr. Polym. 2010, 82, 28–33. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Li, L.; Hou, Y.; Sun, J.; Wang, J. A rapid quantitative method for polysaccharides in green tea and oolong tea. Eur. Food Res. Technol. 2008, 226, 691–696. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, D.; Sun, P.; Bucheli, P.; Li, L.; Hou, Y.; Wang, J. Effects of soluble tea polysaccharides on hyperglycemia in alloxan-diabetic mice. J. Agric. Food. Chem. 2007, 55, 5523–5528. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Li, J.; Zhao, G. Components and activity of polysaccharides from coarse tea. J. Agric. Food. Chem. 2001, 49, 507–510. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Zhang, N.; Chen, S.; Tian, J.; Zhang, Y.; Wang, Z. Extrusion treatment for improved physicochemical and antioxidant properties of high-molecular weight polysaccharides isolated from coarse tea. Food Res. Int. 2013, 53, 726–731. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huo, J.; Zhao, T.; Ren, J.; Wei, X. Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. Int. J. Biol. Macromol. 2013, 62, 714–719. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef]
- Jin, F.; Jia, L.-Y.; Tu, Y.-Y. Structural analysis of an acidic polysaccharide isolated from white tea. Food Sci. Biotechnol. 2015, 24, 1623–1628. [Google Scholar] [CrossRef]
- Xu, R.; Ye, H.; Sun, Y.; Tu, Y.; Zeng, X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem. Toxicol. 2012, 50, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Zhang, J.; Xiao, J.; Wei, X. Study on the purification and characterization of a polysaccharide conjugate from tea flowers. Int. J. Biol. Macromol. 2010, 47, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Mao, F.; Liu, Y.; Wei, X. Purification, characterization and biological activities in vitro of polysaccharides extracted from tea seeds. Int. J. Biol. Macromol. 2013, 62, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, S.; Xu, P.; Chen, H.; Lin-Shiau, S.-Y.; Deng, Y.-T.; Lin, J.-K. Fermentation process enhanced production and bioactivities of oolong tea polysaccharides. Food Res. Int. 2012, 46, 158–166. [Google Scholar] [CrossRef]
- Mao, Y.; Wei, B.; Teng, J.; Xia, N.; Zhao, M.; Huang, L.; Ye, Y. Polysaccharides from Chinese Liupao dark tea and their protective effect against hyperlipidemia. Int. J. Food Sci. Tech. 2018, 53, 599–607. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Wang, Y.; Hochstetter, D.; Zhou, T.; Wang, Y. Oral administration of puerh tea polysaccharides lowers blood glucose levels and enhances antioxidant status in alloxan-induced diabetic mice. J. Food Sci. 2012, 77, H246–H252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, G.; Chen, D.; Ye, H.; Sun, Y.; Zeng, X.; Liu, Z. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Biol. Macromol. 2019, 140, 858–870. [Google Scholar] [CrossRef]
- Scoparo, C.T.; Souza, L.M.; Dartora, N.; Sassaki, G.L.; Santana-Filho, A.P.; Werner, M.F.P.; Borato, D.G.; Baggio, C.H.; Iacomini, M. Chemical characterization of heteropolysaccharides from green and black teas (Camellia sinensis) and their anti-ulcer effect. Int. J. Biol. Macromol. 2016, 86, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Jin, Z. Structure analysis of a neutral polysaccharide isolated from green tea. Food Res. Int. 2009, 42, 739–745. [Google Scholar] [CrossRef]
- Wei, X.; Chen, M.; Xiao, J.; Liu, Y.; Yu, L.; Zhang, H.; Wang, Y. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr. Polym. 2010, 79, 418–422. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Chen, C.; Zeng, X.; Ye, H. Prebiotics effects in vitro of polysaccharides from tea flowers on gut microbiota of healthy persons and patients with inflammatory bowel disease. Int. J. Biol. Macromol. 2020, 158, 968–976. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Meng, H.; Li, W.; Li, Q.; Luo, Y.; Wang, C.; Xie, J.; Wu, L.; Zhang, X.; et al. Characteristics of the emulsion stabilized by polysaccharide conjugates alkali-extracted from green tea residue and its protective effect on catechins. Ind. Crops Prod. 2019, 140, 111611. [Google Scholar] [CrossRef]
- Li, Q.; Shi, J.; Du, X.; McClements, D.J.; Chen, X.; Duan, M.; Liu, L.; Li, J.; Shao, Y.; Cheng, Y. Polysaccharide conjugates from Chin brick tea (Camellia sinensis) improve the physicochemical stability and bioaccessibility of β-carotene in oil-in-water nanoemulsions. Food Chem. 2021, 357, 129714. [Google Scholar] [CrossRef]
- Xiang, L.; Si, C.; Zhao, Z.-T.; Meng, Z.; Yi, H.; Ye, X.-M.; Qi, A.; Ouyang, K.-H.; Wang, W.-J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61–70. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Preparation and characterization of a novel conformed bipolymer paclitaxel-nanoparticle using tea polysaccharides and zein. Carbohydr. Polym. 2016, 146, 52–57. [Google Scholar] [CrossRef]
- Wu, S.; Li, N.; Yang, C.; Yan, L.; Liang, X.; Ren, M.; Yang, L. Synthesis of cationic branched tea polysaccharide derivatives for targeted delivery of siRNA to hepatocytes. Int. J. Biol. Macromol. 2018, 118, 808–815. [Google Scholar] [CrossRef] [PubMed]


| Resource | Processing Technologies or Extraction Methods | Name | Monosaccharide Composition (mol.% or Mole Ratio) | Mw/kDa | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glc | Rha | Ara | Man | Rib | Xyl | Gal | Fuc | GalA | GluA | |||||
| Green tea leaves (Artificially Se-enriched Enshi) | HWE (90 °C) | ASe-TPS2 | 1.00 | 1.93 | 7.05 | 1.05 | 26.12 | 6.73 | [28] | |||||
| Green tea leaves (Naturally Se-enriched Enshi) | NSe-TPS2 | 0.10 | 0.28 | 0.59 | 1.00 | 0.07 | 1.24 | 0.49 | 244.32 | |||||
| Green tea leaves (Se-enriched Enshi) | HWE (70 °C) | Se-TPS | 0.49 | 0.22 | 0.71 | 0.12 | 0.14 | 1.00 | 0.03 | 1.39 | 0.13 | 1.3–1020 | [32] | |
| Se-TPS1 | 0.47 | 0.21 | 0.58 | 1.00 | 0.07 | 1.75 | 0.17 | 110 | ||||||
| Se-TPS2 | 0.10 | 0.28 | 0.59 | 1.00 | 0.07 | 1.24 | 0.49 | 240 | ||||||
| Se-TPS3 | 0.30 | 0.38 | 0.72 | 1.00 | 0.07 | 0.88 | 0.19 | 250–920 | ||||||
| Green tea (Se-enriched Ziyang) | HWE (80 °C) | Se-TP | 32.35 | 1.69 | 30.64 | 3.57 | 25.81 | 2.26 | - | [18] | ||||
| Green tea (Coarse) | WE | WE-CTPS | 29.22 | 4.11 | 9.96 | 4.62 | 3.46 | 28.05 | 4.14 | 16.43 | 3.0–2560 | [16] | ||
| WAE | UAE-CTPS | 36.05 | 2.27 | 9.22 | 4.75 | 5.38 | 27.54 | 6.72 | 8.07 | 3.2–3680 | ||||
| MAE | MAE-CTPS | 31.09 | 4.03 | 11.84 | 6.17 | 3.64 | 27.06 | 6.84 | 9.33 | 3.1–2500 | ||||
| EE | EE-CTPS | 44.24 | 5.40 | 8.86 | 4.38 | 3.15 | 12.32 | 11.78 | 9.87 | 3.0–3150 | ||||
| Green tea (Low grade) | HWE | TPC | 14.10 | 8.74 | 29.04 | 7.11 | 0.42 | 35.89 | 4.69 | 6.62, 48.5, 455 | [33] | |||
| Green tea | NaCl solution extraction | TPSA | 15.2 | 6.7 | 20.0 | 2.1 | 2.5 | 1.3 | 20.3 | 470 | [34] | |||
| Green tea | BWE | GSP | 7 | 2 | 19 | 7 | 65 | - | [35] | |||||
| Green tea (Huangshan Maofeng) | HWE (80 °C) | HMTP | 7.4 | 2.3 | 28.9 | 4.9 | 6.1 | 1.1 | 35.0 | 11.3 | 3.0 | - | [36] | |
| Green tea | - | TF-1 | 1 | 3.2 | 1.4 | 231.6 | [37] | |||||||
| TF-2 | 1 | 1.7 | 46.3 | |||||||||||
| TF-3 | 1 | 0.9 | 2.5 | 7.3 | ||||||||||
| Green tea | NaCl solution extraction | ALTPS | 23.8 | 1.6 | 6.9 | 1.0 | 1.7 | 0.6 | 6.6 | - | [38] | |||
| Green tea | HWE (90 °C) | TPC-W | 14.10 | 8.74 | 29.04 | 7.11 | 0.42 | 35.89 | 4.69 | 6.62–4550 | [39] | |||
| AE | TPC-A | 6.28 | 13.81 | 36.07 | 4.89 | 5.24 | 32.27 | 1.43 | 4.13–4940 | |||||
| Green tea | HWE (70 °C) | GTPS | 17 | 7.8 | 41.8 | 7.3 | 7.1 | 18.7 | 9.2–251.5 | [8] | ||||
| Green tea | HWE (65 °C) with EE | ATPS-2 | 0.68 | 1.00 | 1.58 | 4.43 | [29] | |||||||
| Green tea | HWE (75 °C) | Shufeng | 18.70 | 3.15 | 33.90 | 2.45 | 3.36 | 38.44 | 127 | [40] | ||||
| Longjin D | 17.17 | 3.38 | 32.42 | 2.83 | 2.80 | 41.41 | 106 | |||||||
| Jialaoshan | 16.36 | 3.53 | 32.31 | 3.14 | 2.67 | 42.97 | 121 | |||||||
| Green tea | HWE (75 °C) | TPF | 1.01 | 1 | 18.86 | 5.73 | 2.47 | 18.54 | 1.01 | - | [41] | |||
| Green tea | EE | TPS | 43.27 | 6.49 | 2.60 | 41.11 | 6.53 | 110 | [42] | |||||
| Green tea leaves | BWE | 7WA | 1.0 | 0.96 | 71 | [17] | ||||||||
| Green tea leaves (Coarse) | Untreated | TPSU | 1.00 | 0.88 | 1.19 | 0.34 | 1.00 | 1–330 | [43] | |||||
| Extrusion treatment | TPSE4 | 1.00 | 0.12 | 0.93 | 0.13 | 0.62 | 15–330 | |||||||
| Extrusion treatment | TPSE12 | 1.00 | 0.20 | 1.11 | 0.18 | 0.63 | 4–405 | |||||||
| Green tea leaves | Freeze-drying of TPS | TPS-F | 2.82 | 1.0 | 2.77 | 0.45 | 0.58 | 3.12 | 0.14 | 3.88 | 0.26 | 3.3–952 | [44] | |
| Vacuum-drying of TPS | TPS-V | 5.96 | 1.0 | 2.62 | 0.51 | 0.47 | 1.94 | 0.16 | 3.0 | 0.20 | 3.4–910 | |||
| Spray-drying of TPS | TPS-S | 0.77 | 1.0 | 2.43 | 0.40 | 0.46 | 0.96 | 0.10 | 1.94 | 0.18 | 3.3–969 | |||
| Microwave-vacuum drying of TPS | TPS-M | 1.30 | 1.0 | 1.90 | 0.40 | 0.29 | 1.72 | 0.15 | 1.66 | 0.16 | 3.5–915 | |||
| Green tea leaves | HWE (60 °C) | TPS-1 | 13.6 | 39.4 | 10.2 | 0.3 | 0.5 | 31.0 | 1.3 | 2.1 | 0.9 | 20.8 | [19] | |
| TPS-2 | 1.0 | 4.1 | 36.4 | 0.9 | 2.3 | 43.1 | 0.1 | 6.9 | 5.2 | 24.2 | ||||
| TPS-3 | 7.0 | 3.5 | 13.0 | 0.6 | 0.1 | 0.2 | 22.1 | 0.1 | 49.1 | 3.9 | 250.6 | |||
| TPS-4 | 4.0 | 3.1 | 7.7 | 0.8 | 15.4 | 0.2 | 12.8 | 0.2 | 51.2 | 2.5 | 4.1, 689.1 | |||
| Green tea leaves | HWE | HWE-TLPS | 1.00 | 4.82 | 0.22 | 0.48 | 0.21 | 2.93 | 2.06 | 0.22 | 1.17–413 | [45] | ||
| BWE | BWE-TLPS | 1.00 | 0.50 | 1.04 | 0.22 | 0.06 | 1.38 | 1.48 | 0.09 | 1.04–458 | ||||
| EE | EE-TLPS | 1.00 | 1.09 | 1.80 | 2.27 | 2.36 | 0.12 | 1.02–487 | ||||||
| Green tea leaves | HWE (90 °C) | TLPS | 1.77 | 0.87 | 1.87 | 0.11 | 0.3 | 0.07 | 1.00 | 0.29 | 2.54 | 0.24 | 3.67–758 | [10] |
| Green tea leaves (Xihu Longjing) | HWE (90 °C) | XTPS | 5.52 | 9.50 | 8.79 | 3.52 | 1.24 | 13.17 | 11.60 | 1.26–810 | [11] | |||
| Green tea leaves (Chawentianxia) | CTPS | 11.06 | 8.73 | 11.95 | 3.81 | 1.17 | 16.53 | 15.06 | 12–805 | |||||
| Green tea leaves (Huizhoulvcha) | HTPS | 7.28 | 9.48 | 23.06 | 3.75 | 0.96 | 30.68 | 18.36 | 1.2–771 | |||||
| Green tea leaves (Chinese tea Zhongcha 108) | Hydrothermal extraction | F0 | 7.5 | 33.8 | 2.1 | 13.9 | 1.4 | 41.3 | 51.85 | [5] | ||||
| F0.1 | 22.8 | 46.8 | 3.9 | 26.5 | 40.00 | |||||||||
| F0.2 | 38.3 | 39.7 | 22.0 | 32.72 | ||||||||||
| F0.3 | 44.7 | 36.4 | 18.9 | 25.27 | ||||||||||
| F0.4 | 45.9 | 35.8 | 18.3 | 18.38 | ||||||||||
| White tea leaves | - | WTPS | 2.2 | 1.1 | 4.2 | 4.5 | 1 | 29 | [46] | |||||
| Green tea flowers | HWE (90 °C) | TFPS | 11.54 | 10.17 | 49.52 | 2.68 | 1.49 | 22.04 | 2.58 | - | [47] | |||
| TFPS-1 | 45.39 | 14.84 | 6.87 | 12.16 | 18.08 | 2.64 | - | |||||||
| TFPS-2 | 11.19 | 55.16 | 33.65 | - | ||||||||||
| TFPS-3 | 20.95 | 53.34 | 25.71 | - | ||||||||||
| Green tea flowers | HWE (80 °C) | TFPS-1 | 1.0 | 0.81 | 1.2 | 0.98 | - | [9] | ||||||
| TFPS-2 | 1.0 | 2.3 | 2.3 | 0.76 | 10.1 | |||||||||
| Green tea flowers | HWE (90 °C) | TFPS1 | 1.3 | 1.0 | 2.9 | 0.5 | 3.3 | 500 | [48] | |||||
| Green tea flowers | HWE | HWE-TFPS | 1.00 | 0.36 | 1.19 | 0.23 | 0.16 | 2.09 | 0.25 | 0.12 | 1.06–483 | [45] | ||
| BWE | BWE-TFPS | 1.00 | 0.80 | 2.06 | 0.25 | 0.20 | 2.47 | 2.20 | 0.13 | 1.06–508 | ||||
| EE | EE-TFPS | 1.00 | 1.33 | 2.90 | 0.21 | 0.24 | 3.20 | 2.10 | 0.12 | 1.18–465 | ||||
| Green tea flowers | HWE (90 °C) | TFPS | 0.36 | 0.42 | 0.97 | 0.17 | 0.11 | 1.00 | 0.71 | 0.08 | 2.56–1460 | [10] | ||
| Green tea seeds | Na-citric acid buffer extraction | TSPS | 1.95 | 0.35 | 0.95 | 0.15 | 1.00 | 0.23 | 0.07 | 3.66–961 | [10] | |||
| Green tea seeds | Extracted with Na-citric acid buffer, enzyme and hot water in sequence | NTSPS | 12.44 | 1.16 | 1 | 4588 | [49] | |||||||
| ATSPS-1 | 0.03 | 0.51 | 0.78 | 0.07 | 0.09 | 1 | 0.1 | 0.06 | 500 | |||||
| ATSPS-2 | 23.45 | 0.76 | 0.43 | 1 | 100 | |||||||||
| Oolong tea | Ultrafiltration with Mw >80 kDa | OTPS1 | 7.90 | 5.50 | 7.31 | 5.78 | 9.43 | 10.32 | 13.11 | 11.85 | - | [27] | ||
| Ultrafiltration with Mw 30–80 kDa | OTPS2 | 17.13 | 5.39 | 6.90 | 10.90 | 6.90 | 8.43 | 27.32 | 8.20 | - | ||||
| Ultrafiltration with Mw 10–30 kDa | OTPS3 | 35.94 | 6.56 | 5.22 | 8.39 | 7.01 | 8.98 | 13.35 | 5.29 | - | ||||
| Ultrafiltration with Mw <10 kDa | OTPS4 | 27.86 | 6.13 | 4.99 | 4.16 | 11.79 | 7.39 | 3.65 | 7.35 | - | ||||
| Oolong tea (Tieguanyin) | HWE (70 °C) | TTPS | 26.39 | 5.75 | 26.84 | 2.91 | 0.81 | 35.34 | 1.96 | 25, 25, 817 | [50] | |||
| Oolong tea (Fenghuangdancong) | FTPS | 14.44 | 10.83 | 25.69 | 6.97 | 2.39 | 35.85 | 3.83 | 14, 930 | |||||
| Oolong tea (Dahongpao) | DTPS | 22.59 | 10.31 | 22.93 | 5.21 | 0.28 | 33.59 | 5.09 | 42, 110, 2640 | |||||
| Oolong tea | HWE (70 °C) | OTPS | 21.9 | 16.2 | 43.7 | 18.0 | 5.3–100.9 | [8] | ||||||
| Oolong tea (Anxi Tieguanyin) | HWE (90 °C) | TTPS | 12.74 | 7.41 | 13.78 | 5.7 | 1.37 | 20.16 | 15.49 | 1.2–762 | [11] | |||
| Oolong tea | HWE (75 °C) | Fenghuangdanzong | 14.38 | 3.65 | 31.70 | 2.60 | 2.79 | 44.87 | 107 | [40] | ||||
| Tieguanyin | 10.18 | 5.77 | 32.56 | 1.22 | 3.81 | 46.47 | 95 | |||||||
| Black tea | BWE | BSP | 16 | 3 | 16 | 35 | - | [35] | ||||||
| Black tea | HWE (70 °C) | BTPS | 29.4 | 14.4 | 36.4 | 19.7 | 3.8–32.7 | [8] | ||||||
| Dark tea (Chinese Liubao) | HWE (70 °C) | CLTPS | 0.32 | 3.36 | 3.84 | 2.08 | 1.92 | 0.16 | 467, 11.4 | [51] | ||||
| Puerh tea | Aging time of 1 year | PTPS-1 | 16.52 | 5.34 | 21.86 | 21.59 | 4.04 | 26.93 | 3.64 | 2700 | [12,52] | |||
| Aging time of 3 years | PTPS-2 | 10.23 | 6.82 | 26.22 | 13.83 | 0.35 | 39.34 | 3.21 | 631–1930 | |||||
| Aging time of 5 years | PTPS-3 | 6.08 | 15.98 | 20.84 | 15.29 | 0.15 | 40.33 | 1.68 | 1160–3900 | |||||
| Brick tea (Fuzhuan) | HWE (80 °C) | FBTPS-3 | 15.50 | 13.90 | 8.70 | 19.70 | 42.20 | 741 | [53] | |||||
| Resource | Name | Isolation Methods | Structural Characterization | References |
|---|---|---|---|---|
| Green tea leaves (Artificial Se-enriched Enshi) | ASe-TPS2 | HWE (90 °C)→Ethanol precipitation (60%)→Deproteinization→Dialysis→DEAE Sepharose fast flow gel column |  | [28] |
| Green tea leaves (Natural Se-enriched Enshi) | NSe-TPS2 |  | ||
| Green tea (Zhongcha 108) | F0.3 | WE (120 °C)→Dialysis→Deproteinization→DEAE-52 column |  | [5] |
| Green tea | GTPS | HWE (100 °C)→AE (10% NaOH)→Dialysis→Freeze-thawing | Main chain of (1→3)-β-Galp, substituted at O-6 by (1→6)-linked β-Galp with side chains of α-Araf and terminal units of α-Rhap, α-Fucp and α-Araf | [54] |
| Black tea | BTPS | |||
| Green tea | GTPI | Main chain of (1→4)-β-Xylp, substituted in O-3 by α-Araf, β-Galp and α-Glcp units | ||
| Black tea | BTPI | |||
| Green tea (Wufeng) | 7WA | BWE (100 °C)→Ethanol precipitation (final concentration was 40% and 70%)→DEAE-cellulose column→Superdex-200 column |  | [17] |
| Green tea (Wufeng) | TPS1-2a TPS1-2b | BWE (100 °C)→Ethanol precipitation (final concentration was 40%)→DEAE-cellulose column→Sephacryl S-300 column |  | [23] |
| Green tea Black tea | GSP BSP | BWE (100 °C)→Ethanol precipitation (95%)→Dialysis→Freeze-thawing | Backbone with a long sequence of →4)-6-O-Me-α-D-GalpA-(1→ and the side chains attached to the α-L-Rhap residues | [35] |
| Tea flowers | TFP-1 | HWE (80 °C)→Ethanol precipitation (95%)→Deproteinization→Dialysis→Sephadex G-100 gel column | α-D-Galp, α-L-Rhap, α-D-Glcp, α-D-GalNAcp and α-D-Glcp residues | [9] |
| TFP-2 | α-D-Glcp, α-D-Xylp, α-D-GalNAcp, α-L-Arap and α-L-Rhap residues | |||
| Tea flowers | TFPS-1 | BWE (90 °C)→Ethanol precipitation (95%)→Dialysis→DEAE Sepharose fast flow gel column | Backbone consisted Glu and Gal, branched chain consisted Ara, Gal and Rha | [48] |
| Green tea | NTPS-1 | HWE (65 °C)→EE (cellulose compound enzyme)→Ethanol precipitation (75%)→Anion exchange resin D315 column →Dialysis→DEAE Sepharose fast flow gel column | β-(1→4)-linked galactopyranosyl units | [55] |
| Green tea | ATPS-2 | EE (plant hydrolase)→HWE (60 °C)→Ethanol precipitation (95%)→Anion exchange resin D315 column→DEAE Sepharose fast flow gel column | Backbone with →4)-α-D-GalpA-(1→2)-α-L-Rhap-(1→4)-α-D-GalpA-(1→, consisting of α-1,4-D-galactopyranosyluronan and 1,2-linked rhamnosyl residues | [29] |
| Green tea (Wuyuan) | TGC | - | Main chain consisted of Gal, Glc and Rha by β-(1→3) linkage, while branch chains connected to main chain by β-(1→3)-, β-(1→2)- and β-(2→3)-linkages | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Wu, P.; Zhan, J.; Wang, W.; Shen, J.; Ho, C.-T.; Li, S. Influencing Factors on the Physicochemical Characteristics of Tea Polysaccharides. Molecules 2021, 26, 3457. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113457
Hu T, Wu P, Zhan J, Wang W, Shen J, Ho C-T, Li S. Influencing Factors on the Physicochemical Characteristics of Tea Polysaccharides. Molecules. 2021; 26(11):3457. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113457
Chicago/Turabian StyleHu, Ting, Peng Wu, Jianfeng Zhan, Weixin Wang, Junfeng Shen, Chi-Tang Ho, and Shiming Li. 2021. "Influencing Factors on the Physicochemical Characteristics of Tea Polysaccharides" Molecules 26, no. 11: 3457. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113457