Study of the Synchrotron Photoionization Oxidation of Alpha-Angelica Lactone (AAL) Initiated by O(3P) at 298, 550, and 700 K
Abstract
:1. Introduction
2. Results and Discussion
2.1. Product Identification
2.2. Branching Fractions
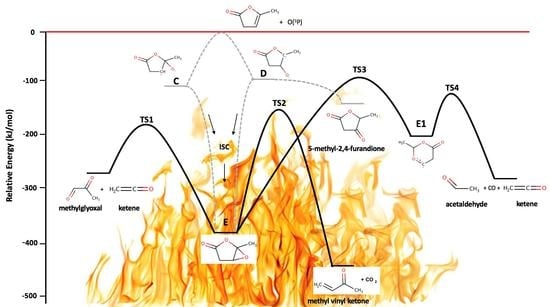
2.3. Reaction Pathways for the Primary Products
3. Methods
Computational Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranzan, L.; Ranzan, C.; Trierweiler, L.F.; Trierweiler, J.O. Classification of Diesel Fuel Using Two-Dimensional Fluorescence Spectroscopy. Energy Fuels 2017, 31, 8942–8950. [Google Scholar] [CrossRef]
- Ramanathan, V.; Feng, Y. Air pollution, greenhouse gases and climate change: Global and regional perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Chang, T.; Zivin, J.S.G.; Gross, T.; Neidell, M. Particulate Pollution and the Productivity of Pear Packers; NBER Working Paper No. 19944; 2014; Available online: https://www.nber.org/papers/w19944 (accessed on 1 April 2020). [CrossRef] [Green Version]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-Fermentative Pathways for Synthesis of Branched-Chain Higher Alcohols as Biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, Economic, and Energetic Costs and Benefits of Biodiesel and Ethanol Biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [Green Version]
- Kohse-Höinghaus, K.; Osswald, P.; Cool, T.A.; Kasper, T.; Hansen, N.; Qi, F.; Westbrook, C.K.; Westmoreland, P.R. Biofuel Combustion Chemistry: From Ethanol to Biodiesel. Angew. Chem. Int. Ed. 2010, 49, 3572–3597. [Google Scholar] [CrossRef]
- Vispute, T.P.; Huber, G.W. Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels. Int. Sugar J. 2008, 110, 138–149. [Google Scholar]
- Malpani, S.; Joseph, E. 2,5-Dimeethylfuran as a Bio-Fuel. J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 71–72. [Google Scholar]
- Mascal, M.; Nikitin, E.B. Direct, High-Yield Conversion of Cellulose into Biofuel. Angew. Chem. Int. Ed. 2008, 47, 7924–7926. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Fathi, Y.; Meloni, G. Study of the Synchrotron Photoionization Oxidation of 2-Methylfuran Initiated by O(3P) under Low-Temperature Conditions at 550 and 650 K. J. Phys. Chem. A 2017, 121, 6966–6980. [Google Scholar] [CrossRef]
- Ritter, S. Race To The Pump. Chem. Eng. News Arch. 2011, 11–12, 14–17. [Google Scholar] [CrossRef]
- Sims, R.; Taylor, M.; Saddler, J.; Mabee, W. From 1st to 2nd-generation biofuel technologies: An overview of current industry and RD&D activities. Int. Energy Agen. 2008. Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-IEAHQ-2nd-generation-Biofuels-Executive-Summary.pdf (accessed on 1 April 2020).
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, P.; Wang, J.; Jiang, P.; Wu, X.; Xue, H.; Liu, J.; Zhou, X.; Li, Q. Production of jet and diesel biofuels from renewable lignocellulosic biomass. Appl. Energy 2015, 150, 128–137. [Google Scholar] [CrossRef]
- Avelino, F.; Silva, K.T.; de Souza Filho, M.d.S.M.; Mazzetto, S.E.; Lomonaco, D. Microwave-assisted organosolv extraction of coconut shell lignin by Brønsted and Lewis acids catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárova, K.; Liptaj, T.; Štolcová, M.; Prónayová, N.; Soták, T. Cyclopentanone: A raw material for production of C15 and C17 fuel precursors. Biomass Bioenergy 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Zaras, A.M.; Dagaut, P.; Serinyel, Z. Computational Kinetic Study for the Unimolecular Decomposition Pathways of Cyclohexanone. J. Phys. Chem. A 2014, 119, 7138–7144. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 2014, 50, 2572. [Google Scholar] [CrossRef]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Hoover, S.W.; Marner, W.D.; Brownson, A.K.; Lennen, R.M.; Wittkopp, T.M.; Yoshitani, J.; Zulkifly, S.; Graham, L.E.; Chaston, S.D.; McMahon, K.D.; et al. Bacterial Production of Free Fatty Acids from Freshwater Macroalgal Cellulose. Appl. Microbiol. Biotechnol. 2011, 91, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Park, E.Y.; Sato, M.; Kojima, S. Fatty Acid Methyl Ester Production Using Lipase-Immobilizing Silica Particles with Different Particle Sizes and Different Specific Surface Areas. Enzym. Microb. Tech. 2006, 39, 889–896. [Google Scholar] [CrossRef]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Etherification and Reductive Etherification of 5-(Hydroxymethyl)Furfural: 5-(Alkoxymethyl)Furfurals and 2,5-Bis(Alkoxymethyl)Furans as Potential Bio-Diesel Candidates. Green Chem. 2012, 14, 1626. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Cannilla, C.; Bonura, G. Catalytic Etherification of Glycerol to Produce Biofuels over Novel Spherical Silica Supported Hyflon Catalysts. Bioresour. Technol. 2012, 118, 350–358. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (Alcohols and Biodiesel) Applications as Fuels for Internal Combustion Engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Atsumi, S. Synthetic Biology Approaches to Produce C3-C6 Alcohols from Microorganisms. Curr. Chem. Biol. 2012, 6, 32–41. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of Dimethylfuran for Liquid Fuels from Biomass-Derived Carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Mehdi, H.; Fábos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horváth, I.T. Integration of Homogeneous and Heterogeneous Catalytic Processes for a Multi-step Conversion of Biomass: From Sucrose to Levulinic Acid, γ-Valerolactone, 1,4-Pentanediol, 2-Methyl-tetrahydrofuran, and Alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Cotton, S. Lactones as Biofuel. 2008. Available online: https://0-edu-rsc-org.brum.beds.ac.uk/soundbite/lactones-as-biofuel/2021245.article (accessed on 1 April 2020).
- Simmie, J.M. Detailed Chemical Kinetic Models for the Combustion of Hydrocarbon Fuels. Prog. Energy Combust. Sci. 2003, 29, 599–634. [Google Scholar] [CrossRef]
- Welz, O.; Zádor, J.; Savee, J.D.; Ng, M.Y.; Meloni, G.; Fernandes, R.X.; Sheps, L.; Simmons, B.A.; Lee, T.S.; Osborn, D.L.; et al. Low-Temperature Combustion Chemistry of Biofuels: Pathways in the Initial Low-Temperature (550 K–750 K) Oxidation Chemistry of Isopentanol. Phys. Chem. Chem. Phys. 2012, 14, 3112. [Google Scholar] [CrossRef]
- Giustini, A.; Meloni, G. Synchrotron Photoionization Study of the Diisopropyl Ether Oxidation. ChemPhysChem 2020, 21, 927–937. [Google Scholar] [CrossRef]
- Shi, Y.; Ge, H.-W.; Brakora, J.L.; Reitz, R.D. Automatic Chemistry Mechanism Reduction of Hydrocarbon Fuels for HCCI Engines Based on DRGEP and PCA Methods with Error Control. Energy Fuels 2010, 24, 1646–1654. [Google Scholar] [CrossRef]
- Cool, T.A.; Nakajima, K.; Mostefaoui, T.A.; Qi, F.; McIlroy, A.; Westmoreland, P.R.; Law, M.E.; Poisson, L.; Peterka, D.S.; Ahmed, M. Selective Detection of Isomers with Photoionization Mass Spectrometry for Studies of Hydrocarbon Flame Chemistry. J. Chem. Phys. 2003, 119, 8356–8365. [Google Scholar] [CrossRef]
- Zádor, J.; Taatjes, C.A.; Fernandes, R.X. Kinetics of Elementary Reactions in Low-Temperature Autoignition Chemistry. Prog. Energy Combust. Sci. 2011, 37, 371–421. [Google Scholar] [CrossRef]
- Lima, C.G.; Monteiro, J.L.; de Melo Lima, T.; Weber Paixão, M.; Corrêa, A.G. Angelica Lactones: From Biomass-Derived Platform Chemicals to Value-Added Products. ChemSusChem 2017, 11, 25–47. [Google Scholar] [CrossRef] [PubMed]
- McAdam, K.; Enos, T.; Goss, C.; Kimpton, H.; Faizi, A.; Edwards, S.; Wright, C.; Porter, A.; Rodu, B. Analysis of Coumarin and Angelica Lactones in Smokeless Tobacco Products. Chem. Cent. J. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Xin, J.; Zhang, Z.; Liu, Z.; Lu, X.; Ren, B.; Zhang, S. Efficient Conversion of α-Angelica Lactone into γ-Valerolactone with Lonic Liquids at Room Temperature. ACS Sustain. Chem. Eng. 2014, 2, 902–909. [Google Scholar] [CrossRef]
- Valco, D.J.; Min, K.; Oldani, A.; Edwards, T.; Lee, T. Low temperature autoignition of conventional jet fuels and surrogate jet fuels with targeted properties in a rapid compression machine. Proc. Combust. Inst. 2017, 36, 3687–3694. [Google Scholar] [CrossRef] [Green Version]
- Cvetanović, R.J. Evaluated Chemical Kinetic Data for the Reactions of Atomic Oxygen O(3P) with Unsaturated Hydrocarbons”. J. Phys. Chem. Ref. Data 1987, 16, 261–326. [Google Scholar] [CrossRef]
- González-Lavado, E.; Corchado, J.C.; Espinosa-Garcia, J. The Hydrogen Abstraction Reaction O(3P) + CH4: A New Analytical Potential Energy Surface Based on Fit to Ab Initio Calculations. J. Chem. Phys. 2014, 140, 064310. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsunaga, F.M.; Sakai, H. Absorption Coefficient and Photoionization Yield of NO in the Region 580–1350 Å. Appl. Opt. 1967, 6, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Goulay, F.; Derakhshan, A.; Maher, E.; Trevitt, A.J.; Savee, J.D.; Scheer, A.M.; Osborn, D.L.; Taatjes, C.A. Formation of Dimethylketene and Methacrolein by Reaction of the CH Radical with Acetone. Phys. Chem. Chem. Phys. 2013, 15, 4049. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wang, J.; Cool, T.A.; Hansen, N.; Skeen, S.; Osborn, D.L. Absolute Photoionization Cross-Sections of Some Combustion Intermediates. Int. J. Mass Spectrom. 2012, 309, 118–128. [Google Scholar] [CrossRef]
- Reed, R.I.; Brand, J.C. Electron Impact Studies. Part 4—Glyoxal, Methylglyoxal, and Diacetyl. Trans. Faraday Soc. 1958, 54, 478–482. [Google Scholar] [CrossRef]
- Thorstad, O.; Undheim, K.; Cederlund, B.; Hörnfeldt, A.-B.; Servin, R.; Sternerup, H. Ionisation Potentials in Tautomeric Analysis of 2-Hydroxy Derivatives of Thiophenes, Selenophenes, and Furans. Acta Chem. Scand. 1975, 29, 647–651. [Google Scholar] [CrossRef]
- Czekner, J.; Taatjes, C.A.; Osborn, D.L.; Meloni, G. Absolute Photoionization Cross-Sections of Selected Furanic and Lactonic Potential Biofuels. Int. J. Mass Spectrom. 2013, 348, 39–46. [Google Scholar] [CrossRef]
- Bobeldijk, M.; van der Zande, W.J.; Kistemaker, P.G. Simple Models for the Calculation of Photoionization and Electron Impact Ionization Cross Sections of Polyatomic Molecules. Chem. Physics 1994, 179, 125–130. [Google Scholar] [CrossRef]
- Cool, T.A.; Wang, J.; Nakajima, K.; Taatjes, C.A.; Mcllroy, A. Photoionization Cross Sections for Reaction Intermediates in Hydrocarbon Combustion. Int. J. Mass Spectrom. 2005, 247, 18–27. [Google Scholar] [CrossRef]
- Heimann, P.A.; Koike, M.; Hsu, C.W.; Blank, D.; Yang, X.M.; Suits, A.G.; Lee, Y.T.; Evans, M.; Ng, C.Y.; Flaim, C.; et al. Performance of the Vacuum Ultraviolet High-Resolution and High-Flux Beamline for Chemical Dynamics Studies at the Advanced Light Source. Rev. Sci. Instrum. 1997, 68, 1945–1951. [Google Scholar] [CrossRef]
- Fathi, Y.; Price, C.; Meloni, G. Low-Temperature Synchrotron Photoionization Study of 2-Methyl-3-buten-2-ol (MBO) Oxidation Initiated by O(3P) Atoms in the 298–650 K Range. J. Phys. Chem. A 2017, 121, 2936–2950. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.W.; Taatjes, C.A.; Welz, O.; Osborn, D.L.; Meloni, G. Synchrotron Photoionization Measurements of OH-Initiated Cyclohexene Oxidation: Ring-Preserving Products in OH+ Cyclohexene and Hydroxycyclohexyl + O2 Reactions. J. Phys. Chem. A 2012, 116, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Bryan, B.M.; Nelson, J.; Meloni, G. Study of tert-Amyl Methyl Ether Low-Temperature Oxidation Using Synchrotron Photoionization Mass Spectrometry. J. Phys. Chem. A 2015, 119, 8667–8682. [Google Scholar] [CrossRef] [PubMed]
- Troe, J. Are Primary Quantum Yields of NO2 Photolysis at λ ≤ 398 nm Smaller Than Unity? Z. Phys. Chem. 2000, 214, 573–581. [Google Scholar] [CrossRef]
- Vandaele, A.C.; Hermans, C.; Simon, P.C.; Carleer, M.; Colin, R.; Fally, S.; Mérienne, M.F.; Jenouvrier, A.; Coquart, B. Measurements of the NO2 Absorption Cross-Section from 42 000 cm−1 to 10 000 cm−1 (238–1000 nm) at 220 K and 294 K. J. Quant. Spectrosc. Radiat. Transf. 1998, 59, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Savee, J.D.; Soorkia, S.; Welz, O.; Selby, T.M.; Taatjes, C.A.; Osborn, D.L. Absolute Photoionization Cross-Section of the Propargyl Radical. J. Chem. Phys. 2012, 136, 134307. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A Complete Basis Set Model Chemistry. VI. Use of Density Functional Geometries and Frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A Complete Basis Set Model Chemistry. VII. Use of the Minimum Population Localization Method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Winfough, M.; Yao, R.; Ng, M.; Catani, K.; Meloni, G. Synchrotron Photoionization Investigation of the Oxidation of Ethyl tert-Butyl Ether. J. Phys. Chem. A 2017, 121, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Duschinsky, F. The importance of the electron spectrum in multi atomic molecules. Concerning the Franck-Condon principle. Acta Physicochim URSS 1937, 7, 551–566. [Google Scholar]
- Sharp, T.E.; Rosenstock, H.M. Franck—Condon Factors for Polyatomic Molecules. J. Chem. Phys. 1964, 41, 3453–3463. [Google Scholar] [CrossRef]
- Lermé, J. Iterative Methods to Compute One- and Two-Dimensional Franck-Condon Factors. Tests of Accuracy and Application to Study Indirect Molecular Transitions. Chem. Phys. 1990, 145, 67–88. [Google Scholar] [CrossRef]
- Ruhoff, P.T. Recursion Relations for Multi-Dimensional Franck-Condon Overlap Integrals. Chem. Phys. 1994, 186, 355–374. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP Density Functional Methods for a Large Set of Organic Molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions—the IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]











| Compound | m/z | 298 K | 550 K | 700 K |
|---|---|---|---|---|
| ketene | 42 | 47.2 ± 14.6 | 39.5 ± 13.4 | 39.7 ± 12.2 |
| acetaldehyde | 44 | 5.6 ± 2.1 | 9.3 ± 4.2 | 15.9 ± 7.1 |
| methyl vinyl ketone | 70 | 34.6 ± 12.6 | 26.7 ± 10.7 | 19.7 ± 7.6 |
| methylglyoxal | 72 | 11.1 ± 3.9 | 7.4 ± 3.6 | 4.7 ± 2.2 |
| dimethylglyoxal | 86 | - | 3.5 ± 1.4 | 3.4 ± 2.0 |
| 5-methyl-2,4-furandione | 114 | 2.2 ± 1.5 | 1.0 ± 0.5 | - |
| Total (C balanced) | 101 ± 19.9 | 87.3 ± 18.1 | 83.4 ± 16.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaei, G.; Meloni, G. Study of the Synchrotron Photoionization Oxidation of Alpha-Angelica Lactone (AAL) Initiated by O(3P) at 298, 550, and 700 K. Molecules 2021, 26, 4070. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26134070
Rezaei G, Meloni G. Study of the Synchrotron Photoionization Oxidation of Alpha-Angelica Lactone (AAL) Initiated by O(3P) at 298, 550, and 700 K. Molecules. 2021; 26(13):4070. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26134070
Chicago/Turabian StyleRezaei, Golbon, and Giovanni Meloni. 2021. "Study of the Synchrotron Photoionization Oxidation of Alpha-Angelica Lactone (AAL) Initiated by O(3P) at 298, 550, and 700 K" Molecules 26, no. 13: 4070. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26134070