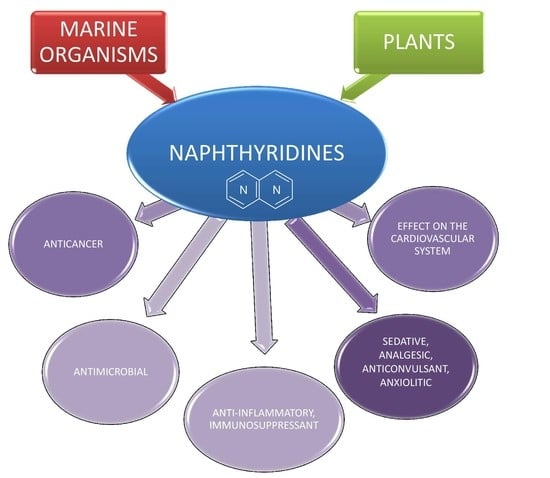
Biological Activity of Naturally Derived Naphthyridines
Abstract
:1. Introduction
2. Naturally Occurring Naphthyridine Derivatives
2.1. 1,5-Naphthyridine Derivatives
2.2. 1,6-Naphthyridine Derivatives
2.3. 1,7-Naphthyridine Derivatives
2.4. 2,6-Naphthyridine Derivatives
2.5. 2,7-Naphthyridine Derivatives
2.5.1. Bicyclic Alkaloids
2.5.2. Tricyclic Alkaloids
2.5.3. Tetracyclic Alkaloids
2.5.4. Pentacyclic Alkaloids
2.5.5. Polycyclic Alkaloids—Pyridoacridine Analogs
Tetracyclic Pyridoacridine Derivatives
Penta- and Hexacyclic Pyridoacridine Derivatives
Hepta- and Oxacyclic Pyridoacridine Derivatives
2.6. Naphthyridines Molecular Mechanisms of Action—What Do We Know?
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hardy, K. Paleomedicine and the evolutionary context of medicinal plant use. Rev. Bras. Farm. 2021, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-naphthyridine derivatives. A new class of chemotherapeutic agents. J. Med. Chem. 1962, 5, 1063–1065. [Google Scholar] [CrossRef]
- Showalter, H.D.H. Progress in the synthesis of canthine alkaloids and ring-truncated congeners. J. Nat. Prod. 2012, 76, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Damazo, A.S.; Macho, A.; Matchado, M.S.; Pavan, E.; Figueiredo, F.D.F.; Oliveira, D.M.; Duckworth, C.A.; Thangaraj, P.; Leonti, M.; et al. Canthin-6-one ameliorates TNBS-induced colitis in rats by modulating inflammation and oxidative stress. An in vivo and in silico approach. Biochem. Pharmacol. 2021, 186, 114490. [Google Scholar] [CrossRef]
- Torquato, H.F.V.; Ribeiro-Filho, A.C.; Buri, M.; Júnior, R.T.A.; Pimenta, R.; de Oliveira, J.S.R.; Filho, V.O.; Macho, A.; Paredes-Gamero, E.J.; Martins, D.T.D.O. Canthin-6-one induces cell death, cell cycle arrest and differentiation in human myeloid leukemia cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In vitro antimicrobial and antiproliferative activities of the root bark extract and isolated chemical constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, J.S.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D.S. A new canthinone-type alkaloid isolated from ailanthus altissima swingle. Molecules 2016, 21, 642. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, J.-K.; Liu, D.; Wang, S.-J.; Wang, J.-R. Synthesis and evaluation of ester derivatives of 10-hydroxycanthin-6-one as potential antimicrobial Agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.E.; Nakayama, H.; de Arias, A.R.; Schinini, A.; de Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on trypanosoma cruzi-infected mice. J. Ethnopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytotherapy Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chang, F.-R.; Wang, H.-K.; Kashiwada, Y.; McPhail, A.T.; Bastow, K.F.; Tachibana, Y.; Cosentino, M.; Lee, K.-H. Anti-HIV agents 451and antitumor agents 205.2 two new sesquiterpenes, leitneridanins A and B, and the cytotoxic and anti-HIV principles from Leitneria floridana. J. Nat. Prod. 2000, 63, 1712–1715. [Google Scholar] [CrossRef]
- Ammirante, M.; Di Giacomo, R.; De Martino, L.; Rosati, A.; Festa, M.; Gentilella, A.; Pascale, M.C.; Belisario, M.A.; Leone, A.; Turco, M.C.; et al. 1-methoxy-canthin-6-one induces c-Jun NH2-terminal kinase–dependent apoptosis and synergizes with tumor necrosis factor–related apoptosis-inducing ligand activity in human neoplastic cells of hematopoietic or endodermal origin. Cancer Res. 2006, 66, 4385–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.; Dirsch, V.M.; Stuppner, H. NF-κB inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Tung, M.H.T.; Đuc, H.V.; Huong, T.T.; Duong, N.T.; Phuong, D.T.; Thao, D.T.; Tai, B.H.; Kim, Y.H.; Bach, T.T.; Cuong, N.M. Cytotoxic compounds from Brucea mollis. Sci. Pharm. 2013, 81, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, W.-H.; Gao, H.; Zhao, F.; Lin, H.-W.; Pan, Y.-M.; Zhou, G.-X.; Yao, X.-S. Anti-inflammatory alkaloids from the stems of Picrasma quassioides Bennet. Chem. Pharm. Bull. 2011, 59, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masdeu, C.; Fuertes, M.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F.; Alonso, C. Fused 1,5-naphthyridines: Synthetic tools and applications. Molecules 2020, 25, 3508. [Google Scholar] [CrossRef]
- Gray, J. Notes on the arrangement of sponges, with the descriptions of some new genera. Proc. Zool. Soc. Lond. 1867, 1867, 492–558. [Google Scholar]
- He, Q.; Miao, S.; Ni, N.; Man, Y.; Gong, K. A review of the secondary metabolites from the marine sponges of the genus aaptos. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Nakamura, H.; Kobayashi, J.; Ohizumi, Y.; Hirata, Y. Isolation and structure of aaptamine a novel heteroaromatic substance possessing α-blocking activity from the sea sponge. Tetrahedron Lett. 1982, 23, 5555–5558. [Google Scholar] [CrossRef]
- Gong, K.; Miao, S.; Yang, L.; Wu, Y.; Guo, J.; Chen, W.; Dai, J.; Du, J.; Xi, S. Aaptamine attenuates the proliferation and progression of non-small cell lung carcinoma. Pharm. Biol. 2020, 58, 1044–1054. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yamanokuchi, R.; Yoshitomi, M.; Sato, K.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.E.; de Voogd, N.; van Soest, R.W.; Yokosawa, H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorg. Med. Chem. Lett. 2010, 20, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Shaari, K.; Ling, K.C.; Rashid, Z.M.; Jean, T.P.; Abas, F.; Raof, S.M.; Zainal, Z.; Lajis, N.H.; Mohamad, H.; Ali, A.M. Cytotoxic aaptamines from malaysian Aaptos aaptos. Mar. Drugs 2008, 7, 1–8. [Google Scholar] [CrossRef]
- Li, Q.-L.; Zhang, P.-P.; Wang, P.-Q.; Yu, H.-B.; Sun, F.; Hu, W.-Z.; Wu, W.-H.; Zhang, X.; Chen, F.; Chu, Z.-Y.; et al. The cytotoxic and mechanistic effects of aaptamine on hepatocellular carcinoma. Anti Cancer Agents Med. Chem. 2015, 15, 291–297. [Google Scholar] [CrossRef]
- Bowling, J.J.; Pennaka, H.K.; Ivey, K.; Wahyuono, S.; Kelly, M.; Schinazi, R.F.; Valeriote, F.A.; Graves, D.E.; Hamann, M.T. Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine. Chem. Biol. Drug Des. 2008, 71, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kong, D.; Suna, H.; Sowa, Y.; Sakai, T.; Setiawan, A.; Kobayashi, M. Aaptamine, a spongean alkaloid, activates p21 promoter in a p53-independent manner. Biochem. Biophys. Res. Commun. 2006, 342, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef]
- Ohizumi, Y.; Kajiwara, A.; Nakamura, H.; Kobayashi, J. α-Adrenoceptor blocking action of aaptamine, a novel marine natural product, in vascular smooth muscle. J. Pharm. Pharmacol. 2011, 36, 785–786. [Google Scholar] [CrossRef]
- Amin, N.M.; Hamdin, M.S.; Amir, M.; Azarnudin, T.; Asari, A.; Nur, F.; Rashid, A.A.; Mariam, S.; Nor, M. Cytotoxicity effect of Aaptamine and its derivatives on Acanthamoeba castellanii (IMR isolate). Sci. Int. 2018, 30, 309–313. [Google Scholar]
- Loffina, D.I.; Volkovitskaya, O.É.; Utkina, N.K.; Fedoreev, S.A. Aaptamine—New selective inhibitor of type A monoamine oxidase. Pharm. Chem. J. 1990, 24, 456–458. [Google Scholar]
- Dyshlovoy, S.A.; Fedorov, S.N.; Shubina, L.K.; Kuzmich, A.S.; Bokemeyer, C.; Amsberg, G.K.-V.; Honecker, F. Aaptamines from the marine sponge Aaptos sp. Display anticancer activities in human cancer cell lines and modulate AP-1-, NF-κB-, and p53-dependent transcriptional activity in mouse JB6 Cl41 cells. BioMed Res. Int. 2014, 8, 469309. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-F.; Lee, M.-G.; El-Shazly, M.; Lai, K.-H.; Ke, S.-C.; Su, C.-W.; Shih, S.-P.; Sung, P.-J.; Hong, M.-C.; Wen, Z.-H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- Shubina, L.K.; Makarieva, T.N.; Dyshlovoy, S.; Fedorov, S.; Dmitrenok, P.S.; Stonik, V. Three new aaptamines from the marine sponge Aaptos sp. and their proapoptotic properties. Nat. Prod. Commun. 2010, 5, 1881–1884. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Tang, X.; Li, P.; Li, G. Suberitine A–D, Four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides. Org. Lett. 2012, 14, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Matsumoto, Y.; Phan, C.-S.; Kamada, T.; Onitsuka, S.; Okamura, H.; Iwagawa, T.; Arima, N.; Tani, F.; Vairappan, C.S. Aaptamine-related alkaloid from the marine sponge Aaptos aaptos. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Yu, H.-B.; Yang, F.; Sun, F.; Ma, G.-Y.; Gan, J.-H.; Hu, W.-Z.; Han, B.-N.; Jiao, W.-H.; Lin, H.-W. Cytotoxic aaptamine derivatives from the South China sea sponge Aaptos aaptos. J. Nat. Prod. 2014, 77, 2124–2129. [Google Scholar] [CrossRef]
- Wang, P.; Huang, J.; Kurtán, T.; Mándi, A.; Jia, H.; Cheng, W.; Lin, W. Aaptodines A–D, Spiro naphthyridine–furooxazoloquinoline hybrid alkaloids from the Sponge Aaptos suberitoides. Org. Lett. 2020, 22, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Efferth, T.; Singab, A.N.B. The pharmacology of the genus Sophora (Fabaceae): An updated review. Phytomedicine 2019, 64, 153070. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, J.; Song, D.; Li, Q.; Li, L.; Li, B. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol. Med. Rep. 2020, 22, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Du, Z.; Fang, Z.; Cheng, H.; Li, C.; Zhou, X. Matrine induces apoptosis and autophagy in human lung adenocarcinoma Cells via upregulation of Cavin3 and suppression of PI3K/AKT pathway. J. BUON 2020, 25, 1512–1516. [Google Scholar]
- Liang, X.; Ju, J. Matrine inhibits ovarian cancer cell viability and promotes apoptosis by regulating the ERK/JNK signaling pathway via p38MAPK. Oncol. Rep. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Zhao, B.; Li, B.; Liu, Q.; Gao, F.; Zhang, Z.; Bai, H.; Wang, Y. Effects of matrine on the proliferation and apoptosis of vincristine-resistant retinoblastoma cells. Exp. Ther. Med. 2020, 20, 2838–2844. [Google Scholar] [CrossRef]
- Chen, Z.; Nishimura, N.; Okamoto, T.; Wada, K.; Naora, K. Molecular mechanism of matrine from Sophora alopecuroides in the reversing effect of multi-anticancer drug resistance in K562/ADR cells. BioMed Res. Int. 2019, 2019, 1269532. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Huang, J.; Liu, Y.; Zhao, Y.; Wei, M. Matrine protects cardiomyocytes against hyperglycemic stress by promoting mitofusin 2-induced mitochondrial fusion. Front. Physiol. 2021, 11, 597429. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Zhang, J. Protective role of matrine in sepsis-associated cardiac dysfunction through regulating the lncRNA PTENP1/miR-106b-5p axis. Biomed. Pharmacother. 2021, 134, 111112. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Lin, X.-T.; Zeng, K.-L.; Zhang, L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobil. Pancreat. Dis. Int. 2004, 3, 69–72. [Google Scholar]
- Lao, Y. Clinical study on effect of matrine injection to protect the liver function for patients with primary hepatic carcinoma after trans-artery chemo-embolization (TAE). J. Chin. Med. Mater. 2005, 28, 637–638. [Google Scholar]
- Xu, X.; Ling, Q.; Gao, F.; He, Z.-L.; Xie, H.-Y.; Zheng, S.-S. Hepatoprotective effects of marine and kuhuang in liver transplant recipients. Am. J. Chin. Med. 2009, 37, 27–34. [Google Scholar] [CrossRef]
- Chu, Y.-J.; Ma, W.-D.; Thome, R.; Ping, J.-D.; Liu, F.-Z.; Wang, M.-R.; Zhang, M.-L.; Zhang, G.; Zhu, L. Matrine inhibits CNS autoimmunity through an IFN-β-dependent mechanism. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Dou, M.; Zhou, X.; Li, L.; Zhang, M.; Wang, W.; Wang, M.; Jing, Y.; Ma, R.; Zhao, J.; Zhu, L. Illumination of molecular pathways in multiple sclerosis lesions and the immune mechanism of matrine treatment in EAE, a mouse model of MS. Front. Immunol. 2021, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, H.; Sun, P.; Khan, A.; Guo, J.; Zheng, X.; Sun, Y.; Fan, K.; Yin, W.; Li, H. Matrine exhibits antiviral activity in a PRRSV/PCV2 co-infected mouse model. Phytomedicine 2020, 77, 153289. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Sun, P.; Lv, H.; Sun, Y.; Guo, J.; Wang, Z.; Luo, T.; Wang, S.; Li, H. Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type. Sci. Rep. 2016, 6, 24401. [Google Scholar] [CrossRef]
- Kianbakht, S.; Hajiaghaee, R.; Akhondzadeh, S. Efficacy and safety of Sophora alopecuroides var. alopecuroides seed extract for opioid detoxification: A randomized, double-blind, and placebo-controlled clinical trial. Phytotherapy Res. 2019, 34, 1108–1113. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, C.-F.; Liu, X.-S.; Jiang, J. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens ait. Basic Clin. Pharmacol. Toxicol. 2010, 108, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Li, Y.; Xiao, Z.; Hu, Z.; Zhang, J. Sophocarpine and matrine inhibit the production of TNF-α and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int. Immunopharmacol. 2008, 8, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Yamada, K.; Kimura, M.; Hamada, M. Nematicidal activity of matrine and its derivatives against pine wood nematodes. J. Agric. Food Chem. 1991, 39, 189–191. [Google Scholar] [CrossRef]
- Tang, Q.; Luo, D.; Lin, D.-C.; Wang, W.-Z.; Li, C.-J.; Zhuo, X.-F.; Wu, Z.-N.; Zhang, Y.-B.; Wang, G.-C.; Li, Y.-L. Five matrine-type alkaloids from Sophora tonkinensis. J. Nat. Med. 2021, 75, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-C.; Dai, W.-F.; Liu, D.; Zhang, Z.-J.; Jiang, M.-Y.; Rao, K.-R.; Li, R.-T.; Li, H.-M. Quinolizidine alkaloids from Sophora alopecuroides with anti-inflammatory and anti-tumor properties. Bioorg. Chem. 2021, 110, 104781. [Google Scholar] [CrossRef]
- Fan, C.-L.; Zhang, Y.-B.; Chen, Y.; Xie, P.; Wang, G.-C.; Tian, H.-Y.; Li, Y.-L.; Huang, X.-J.; Zhang, X.-Q.; Li, Z.-Y.; et al. Alopecuroides A–E, matrine-type alkaloid dimers from the aerial parts of Sophora alopecuroides. J. Nat. Prod. 2019, 82, 3227–3232. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhu, Z.; Hao, T.-T.; Fang, Q.-Q.; Jiang, K.; Qu, S.-J.; Zuo, J.-P.; Zhu, W.; He, S.-J.; Tan, C.-H. Alopecines A–E, five chloro-containing matrine-type alkaloids with immunosuppressive activities from the seeds of Sophora alopecuroides. Bioorg. Chem. 2020, 99, 103812. [Google Scholar] [CrossRef]
- Pan, Q.-M.; Li, Y.-H.; Hua, J.; Huang, F.-P.; Wang, H.-S.; Liang, D. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015, 78, 1683–1688. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Shoji, T.; Arai, T.; Nugroho, A.E.; Deguchi, J.; Hosoya, T.; Uchiyama, N.; Goda, Y.; Awang, K.; Hadi, A.H.A.; et al. Bisleuconothine A, an eburnane–aspidosperma bisindole alkaloid from Leuconotis griffithii. Bioorg. Med. Chem. Lett. 2010, 20, 2021–2024. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-M.; Feng, T.; Wang, Y.-Y.; Li, X.-Y.; Ye, Z.; An, T.; Qing, C.; Luo, X.-D.; Li, Y. Bisleuconothine A, a bisindole alkaloid, inhibits colorectal cancer cell in vitro and in vivo targeting Wnt signaling. Oncotarget 2016, 7, 10203–10214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.P.; Seki, A.; Horiguchi, K.; Shoji, T.; Arai, T.; Nugroho, A.E.; Hirasawa, Y.; Sato, F.; Kaneda, T.; Morita, H. Bisleuconothine A induces autophagosome formation by interfering with AKT-mTOR signaling pathway. J. Nat. Prod. 2015, 78, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, P.; Sun, Q. Bisleuconothine A protects periodontal tissue via the regulation of RANKL expression and infiltration of inflammatory cells. Trop. J. Pharm. Res. 2020, 19, 497–503. [Google Scholar] [CrossRef]
- Li, X.-L.; Xu, M.-J.; Zhao, Y.-L.; Xu, J. A Novel Benzo[f][1,7]Naphthyridine Produced by Streptomyces Albogriseolus from Mangrove Sediments. Molecules 2010, 15, 9298–9307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, C.; Jiao, X.; Liu, X.; Li, R.; Dong, L.; Liu, X.; Zhang, Z.; Xu, J.; Xu, M.; Xie, P. First total synthesis and determination of the absolute configuration of 1-N-methyl-3-methylamino-[N-butanoicacid-3-(9-methyl-8-propen-7-one)-amide]-benzo[f][1,7]naphthyridine-2-one, a novel benzo naphthyridine alkaloid. Tetrahedron Lett. 2012, 53, 4892–4895. [Google Scholar] [CrossRef]
- Harkiss, K.; Swift, D. 4-methyl-2, 6-naphthyridine, a new plant constituent from antirrhinum majus. Tetrahedron Lett. 1970, 11, 4773–4774. [Google Scholar] [CrossRef]
- Padwa, A.; Hennig, R.; Kappe, A.C.O.; Reger, T.S. A triple cascade sequence as a strategy for the construction of the erythrinane skeleton. J. Org. Chem. 1998, 63, 1144–1155. [Google Scholar] [CrossRef]
- Tsuda, Y.; Sano, T. Chapter 4 erythrina and related Alkaloids. In Alkaloids: Chemistry and Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 1996. [Google Scholar]
- Dagne, E.; Steglich, W. Erymelanthine, a new type of erythrina alkaloid containing a 16-azaerythrinane skeleton. Tetrahedron Lett. 1983, 24, 5067–5070. [Google Scholar] [CrossRef]
- Jackson, A.H. The Chemistry and Biology of Isoquinoline Alkaloids; Phillipson, J.D., Roberts, M.F., Zenk, M.H., Eds.; Springer: Berlin, Germany, 1985; p. 75. [Google Scholar]
- Ozawa, M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Ohsaki, A. TRAIL-enhancing activity of erythrinan alkaloids from Erythrina velutina. Bioorg. Med. Chem. Lett. 2009, 19, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Redha, F.J.M. Chromatographic and Spectroscopic Studies of Erythrina Alkaloids. Ph.D. Thesis, University of Wales Cardiff, Cardiff, UK, 1983. [Google Scholar]
- Chawla, A.S.; Kapoor, V.K. Erythrina Alkaloids. Alkaloids Chem. Biol. Perspect. 1995, 9, 85–153. [Google Scholar] [CrossRef]
- Gordin, H.M. On the crystalline alkaloid of calycanthus glaucus. Third paper—On isocalycanthine, isomeric with calycanthine. J. Am. Chem. Soc. 1909, 31, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Manske, R.H.F. Calycanthine I. the isolation of calycanthine from meratia praecox. J. Am. Chem. Soc. 1929, 51, 1836–1839. [Google Scholar] [CrossRef]
- Chen, A.L.; Powell, C.E.; Chen, K.K. The action of calycanthine. J. Am. Pharm. Assoc. 1942, 31, 513–516. [Google Scholar] [CrossRef]
- Chebib, M. Convulsant actions of calycanthine. Toxicol. Appl. Pharmacol. 2003, 190, 58–64. [Google Scholar] [CrossRef]
- Verotta, L.; Pilati, T.; Tatò, M.; Elisabetsky, E.; Amador, T.A.; Nunes, D.S. Pyrrolidinoindoline alkaloids from Psychotria colorata. J. Nat. Prod. 1998, 61, 392–396. [Google Scholar] [CrossRef]
- Adjibade, Y.; Weniger, B.; Quirion, J.C.; Kuballa, B.; Cabalion, P.; Anton, R. Dimeric alkaloids from Psychotria forsteriana. Phytochemistry 1992, 31, 317–319. [Google Scholar] [CrossRef]
- Kitajima, M.; Mori, I.; Arai, K.; Kogure, N.; Takayama, H. Two new tryptamine-derived alkaloids from Chimonanthus praecox f. concolor. Tetrahedron Lett. 2006, 47, 3199–3202. [Google Scholar] [CrossRef]
- Woodward, R.B.; Yang, N.C.; Katz, T.J.; Clark, V.M.; Harley-Mason, J.; Ingleby, R.F.J. Calycanthine: The structure of the alkaloid and its degradation product, calycanine. Proc. Chem. Soc. 1960, 2, 76–78. [Google Scholar]
- Zhang, J.-W.; Gao, J.-M.; Xu, T.; Zhang, X.-C.; Ma, Y.-T.; Jarussophon, S.; Konishi, Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014, 68, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Bennasar, M.-L.; Roca, T.; Zulaica, E.; Monerris, M. Total synthesis of the naphthyridine alkaloid jasminine. Tetrahedron 2004, 60, 6785–6789. [Google Scholar] [CrossRef]
- Hart, N.K.; Johns, S.R.; Lamberton, J.A. The isolation of the alkaloid jasminixe from Olea paniculata (Oleaceae). Aust. J. Chem. 1971, 24, 1739–1740. [Google Scholar] [CrossRef]
- Collins, D.J.; Culvenor, C.C.J.; Lamberton, J.A.; Loder, J.W.; Price, J.R. Plants for Medicines: A Chemical and Pharmacological Survey of Plants in the Australian Region; CSIRO Australia: Melbourne, Australia, 1990. [Google Scholar]
- Ripperger, H. Jasminidin, ein neues monoterpene alkaloid aus Syringa vulgaris. Phytochemistry 1978, 17, 1069. [Google Scholar] [CrossRef]
- Benkrief, R.; Ranarivelo, Y.; Skaltsounis, A.-L.; Tillequin, F.; Koch, M.; Pusset, J.; Sévenet, T. Monoterpene alkaloids, iridoids and phenylpropanoid glycosides from Osmanthus austrocaledonica. Phytochemistry 1998, 47, 825–832. [Google Scholar] [CrossRef]
- Tiwara, K.P.; Minocha, P.K.; Masood, M.A. Closely related alkaloid, acanthicifoline, was isolated from Acanthus ilicifolius (Acanthaceae). Pol. J. Chem. 1980, 54, 857–858. [Google Scholar]
- Powell, R.G.; Smith, C.R.; Madrigal, R.V. Antitumor activity of Sesbania vesicaria, S. punicea, and S. drummondii seed extracts. Planta Med. 1976, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.G.; Smith, C.R.; Weisleder, D.; Muthard, D.A.; Clardy, J. Sesbanine, a novel cytotoxic alkaloid from Sesbania drummondii. J. Am. Chem. Soc. 1979, 101, 2784–2785. [Google Scholar] [CrossRef]
- Powell, R.G.; Smith, C.R., Jr. An investigation of the antitumor activity of Sesbania drummondii. J. Nat. Prod. 1981, 44, 86–90. [Google Scholar] [CrossRef]
- Janot, M.M.; Guilhem, J.; Contz, O.; Venera, E. Contribution to the study of valerian alcaloids (Valeriana officinalis, L.): Actinidine and naphthyridylmethylketone, a new alkaloid. Ann. Pharm. Fr. 1979, 37, 413–420. [Google Scholar]
- Rahman, A.; Vohra, I.I.; Choudhary, M.I.; de Silva, L.B.; Herath, W.H.M.W.; Navaratne, K.M. Neozeylanicine: A novel alkaloid from the timber of Neonauclea zeylanica. Planta Med. 1988, 54, 461–462. [Google Scholar]
- Gross, H.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Ballantine, D.L.; Murray, T.F.; Valeriote, F.A.; Gerwick, W.H. Lophocladines, bioactive alkaloids from the red Alga lophocladia sp. J. Nat. Prod. 2006, 69, 640–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochanowska-Karamyan, A.J.; Araujo, H.C.; Zhang, X.; El-Alfy, A.; Carvalho, P.; Avery, M.A.; Holmbo, S.D.; Magolan, J.; Hamann, M.T. Isolation and synthesis of veranamine, an antidepressant lead from the marine sponge Verongula rigida. J. Nat. Prod. 2020, 83, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Reifer, I.; White, E.P. Studies on perloline; Perlolidine and some fluorescent derivatives of perloline. N. Z. J. Sci. Technol. B Gen. Sect. 1945, 27, 242–247. [Google Scholar]
- Rizk, A.-F. Poisonous Plant Contamination of Edible Plants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Nilar, N.; Sidebottom, P.J.; Carté, B.K.; Butler, M.S. Three new pyridoacridine type alkaloids from a Singaporean ascidian. J. Nat. Prod. 2002, 65, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Lotter, M. Short total synthesis of the marine alkaloid subarine. Sci. Pharm. 2009, 77, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bijeire, L.; Legentil, L.; Bastide, J.; Darro, F.; Rochart, C.; Delfourne, E. A total synthesis of subarine, a marine alkaloid related to the pyridoacridine family. Eur. J. Org. Chem. 2004, 2004, 1891–1893. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: East Lansing, MI, USA, 1956. [Google Scholar]
- Shravya, S.; Vinod, B.N.; Sunil, C. Pharmacological and phytochemical studies of Alangium salvifolium Wang.—A review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 217–222. [Google Scholar] [CrossRef]
- Wiart, C. Lead Compounds from Medicinal Plants for the Treatment of Cancer; Academic Press Inc.: Cambridge, MA, USA, 2012. [Google Scholar]
- Pakrashi, S.; Achari, B.; Ali, E.; Dastidar, P.G.; Sinha, R. Novel benzopyridoquinolizine bases from Alangium lamarckii Thw. Tetrahedron Lett. 1980, 21, 2667–2670. [Google Scholar] [CrossRef]
- Jahangir; Brook, M.A.; MacLean, D.B.; Holland, H.L. 8H-Isoquino[2,1-b][2,7]naphthyridin-8-ones: Synthesis of the alangium alkaloids, alangimaridine and alangimarine. Can. J. Chem. 1987, 65, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Jahangir; Maclean, D.B.; Holland, H.L. Preparation of 8-methylberberines and Aza analogues: Synthesis of alamaridine. Can. J. Chem. 1987, 65, 727–733. [Google Scholar] [CrossRef]
- Bhattacharjya, A.; Mukhopadhyay, R.; Pakrashi, S. Structure and synthesis of alamaridine, a novel benzopyridoquinolizine alkaloid from Alangium lamarck II. Tetrahedron Lett. 1986, 27, 1215–1216. [Google Scholar] [CrossRef]
- Carroll, A.; Taylor, W.; Carroll, A. Constituents of eupomatia species. XIII. The structures of new lignans From the tubers and aerial parts of Eupomatia bennettii. Aust. J. Chem. 1991, 44, 1627–1633. [Google Scholar] [CrossRef]
- Kitahara, Y.; Onikura, H.; Shibano, Y.; Watanabe, S.; Mikami, Y.; Kubo, A. Synthesis of eupomatidines 1, 2 and 3 and related compounds including amino-quinoline-quinone structure. Tetrahedron 1997, 53, 6001–6010. [Google Scholar] [CrossRef]
- Khan, M.; Kihara, M.; Omoloso, A. Antimicrobial activity of the alkaloidal constituents of the root bark of Eupomatia laurina. Pharm. Biol. 2003, 41, 277–280. [Google Scholar] [CrossRef]
- Muhammad, I.; Dunbar, D.C.; Takamatsu, S.; Walker, L.A.; Clark, A.M. Antimalarial, cytotoxic, and antifungal alkaloids from Duguetia hadrantha. J. Nat. Prod. 2001, 64, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.U.M.; Giri, G.S.; Hanumaiah, T.; Rao, K.V.J. Sampangine, a New Alkaloid from Cananga odorata. J. Nat. Prod. 1986, 49, 346–347. [Google Scholar] [CrossRef]
- Liu, S.C.; Oguntimein, B.; Hufford, C.D.; Clark, A.M. 3-Methoxysampangine, a novel antifungal copyrine alkaloid from Cleistopholis patens. Antimicrob. Agents Chemother. 1990, 34, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lúcio, A.S.S.C.; Almeida, J.R.G.D.S.; Barbosa-Filho, J.M.; Pita, J.C.L.R.; Branco, M.V.S.C.; Diniz, M.D.F.F.M.; Agra, M.D.F.; Da-Cunha, E.V.; da Silva, M.S.; Tavares, J.F. Azaphenanthrene alkaloids with antitumoral activity from Anaxagorea dolichocarpa sprague & sandwith (Annonaceae). Molecules 2011, 16, 7125–7131. [Google Scholar] [CrossRef] [Green Version]
- Pan, E.; Cao, S.; Brodie, P.J.; Callmander, M.W.; Randrianaivo, R.; Rakotonandrasana, S.; Rakotobe, E.; Rasamison, V.E.; TenDyke, K.; Shen, Y.; et al. Isolation and synthesis of antiproliferative eupolauridine alkaloids of Ambavia gerrardiifrom the madagascar dry forest(1). J. Nat. Prod. 2011, 74, 1169–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluza, J.; Clark, A.M.; Bailly, C. Apoptosis induced by the alkaloid sampangine in HL-60 leukemia cells: Correlation between the effects on the cell cycle progression and changes of mitochondrial potential. Ann. N. Y. Acad. Sci. 2003, 1010, 331–334. [Google Scholar] [CrossRef]
- Kluza, J.; Mazinghien, R.; Degardin, K.; Lansiaux, A.; Bailly, C. Induction of apoptosis by the plant alkaloid sampangine in human HL-60 leukemia cells is mediated by reactive oxygen species. Eur. J. Pharmacol. 2005, 525, 32–40. [Google Scholar] [CrossRef]
- Nasiri, H.R.; Hohmann, K.; Hatemler, M.G.; Plodek, A.; Bracher, F.; Schwalbe, H. In vitro production of reactive oxygen species (ROS) by sampangine. Med. Chem. Res. 2017, 26, 1170–1175. [Google Scholar] [CrossRef]
- Oanh, N.T.T.; Tung, T.H.; Hue, C.T.; Thanh, L.N.; Thao, D.T.; Hang, N.T.M. Two new alkaloids from Polyalthia nemoralis. Nat. Prod. Commun. 2017, 12, 1025–1027. [Google Scholar] [CrossRef] [Green Version]
- Orabi, K.Y.; Clark, A.M.; Hufford, C.D. Microbial transformation of benzosampangine. J. Nat. Prod. 2000, 63, 396–398. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Xu, T.; Jacob, M.R.; Feng, Q.; Lorenz, M.C.; Walker, L.A.; Clark, A.M. Role of heme in the antifungal activity of the azaoxoaporphine alkaloid sampangine. Eukaryot. Cell 2007, 7, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, J.R.; Zjawiony, J.; Liu, S.; Hufford, C.D.; Clark, A.M.; Rogers, R.D. Copyrine alkaloids: Synthesis, spectroscopic characterization, and antimycotic/antimycobacterial activity of A- and B-ring-functionalized sampangines. J. Med. Chem. 1992, 35, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, M.; Cavé, A. Alkaloids of annonaceae. XIV. Identification of canangine and eupolauridine, new alkaloids with a naphthyridine nucleus. Lloydia 1976, 39, 459–460. [Google Scholar] [PubMed]
- Bowden, B.; Ritchie, E.; Taylor, W. Constituents of eupomatia species. II. isolation and structure determination of further eupomatenoid lignans from the bark of Eupomatia laurina. Aust. J. Chem. 1972, 25, 2659–2669. [Google Scholar] [CrossRef]
- Hufford, C.D.; Liu, S.; Clark, A.M.; Oguntimein, B.O. Anticandidal activity of eupolauridine and onychine, alkaloids from Cleistopholis patens. J. Nat. Prod. 1987, 50, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Mukhtar, M.R.; Hadi, A.H.A.; Awang, K.; Mustafa, M.R.; Zaima, K.; Morita, H.; Litaudon, M. Naucline, a new indole alkaloid from the bark of Nauclea officinalis. Molecules 2012, 17, 4028–4036. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, M.R.; Osman, N.; Awang, K.; Hazni, H.; Qureshi, A.K.; Hadi, A.H.A.; Zaima, K.; Morita, H.; Litaudon, M. Neonaucline, a new indole alkaloid from the leaves of Ochreinauclea maingayii (Hook. f.) Ridsd. Molecules 2011, 17, 267–274. [Google Scholar] [CrossRef]
- Ai, Y.; He, H.; Chen, P.; Yan, B.; Zhang, W.; Ding, Z.; Li, D.; Chen, J.; Ma, Y.; Cao, Y.; et al. An alkaloid initiates phosphodiesterase 3A–schlafen 12 dependent apoptosis without affecting the phosphodiesterase activity. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Massiot, G.; Thépenier, P.; Jacquier, M.-J.; Le Men-Olivier, L.; Verpoorte, R.; Delaude, C. Alkaloids of Strychnos johnsonii. Phytochemistry 1987, 26, 2839–2846. [Google Scholar] [CrossRef]
- Taher, M.; Shaari, S.S.; Susanti, D.; Arbain, D.; Zakaria, Z.A. Genus ophiorrhiza: A review of its distribution, traditional uses, phytochemistry, biological activities and propagation. Molecules 2020, 25, 2611. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Pereira, A. A new indole alkaloid from Sarcocephalus latifolius. Heterocycles 1998, 48, 885. [Google Scholar] [CrossRef]
- Caprasse, M.; Houssier, C. Do planar alkaloids from Strychnos usambarensis intercalate into the DNA helix? Biochimie 1983, 65, 157–167. [Google Scholar] [CrossRef]
- Lederer, E.; Teissier, G.; Huttrer, C. The isolation and chemical composition of calliactine, pigment of the sea anemone “Sagartia parasitica” (Calliactis effoeta). Bull. Soc. Chim. Fr. 1940, 7, 608–615. [Google Scholar]
- Molinski, T.F. Marine pyridoacridine alkaloids: Structure, synthesis, and biological chemistry. Chem. Rev. 1993, 93, 1825–1838. [Google Scholar] [CrossRef]
- Delfourne, E.; Kiss, R.; Le Corre, L.; Dujols, F.; Bastide, J.; Collignon, F.; Lesur, B.; Frydman, A.; Darro, F. Synthesis and in vitro antitumor activity of ring C and D-substituted phenanthrolin-7-one derivatives, analogues of the marine pyridoacridine alkaloids ascididemin and meridine. Bioorg. Med. Chem. 2004, 12, 3987–3994. [Google Scholar] [CrossRef]
- Bontemps, N.; Bry, D.; Lopez-Legentil, S.; Simon-Levert, A.; Long, C.; Banaigs, B. Structures and antimicrobial activities of pyridoacridine alkaloids isolated from different chromotypes of the ascidian Cystodytes dellechiajei. J. Nat. Prod. 2010, 73, 1044–1048. [Google Scholar] [CrossRef]
- Skyler, D.; Heathcock, C.H. The pyridoacridine family tree: A useful scheme for designing synthesis and predicting undiscovered natural products. J. Nat. Prod. 2002, 65, 1573–1581. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.; Wálchli, M.R.; Nakamura, H.; Hirata, Y.; Takuma Sasaki, L.; Ohizumi, Y. Cystodytins A, B, and C, novel tetracyclic alkaloids with potent antineoplastic from the Okinawan tunicate Cystodytes dellechiajei. J. Org. Chem. 1988, 53, 1800–1804. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Tanabe, A.; Ishibashi, M.; Cheng, J.-F.; Yamamura, S.; Sasaki, T. Cystodytins D-I, new cytotoxic tetracyclic aromatic alkaloids from the Okinawan marine tunicate Cystodytes dellechiajei. J. Nat. Prod. 1991, 54, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Eldredge, G.S.; Barrows, L.R.; Ireland, C.M. Inhibition of topoisomerase II catalytic activity by pyridoacridine alkaloids from a Cystodytes sp. ascidian: A mechanism for the apparent intercalator-induced inhibition of topoisomerase II. J. Med. Chem. 1994, 37, 3819–3827. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.; Lambert, G.; Babcock, R.C.; Copp, B.R. Isodiplamine, cystodytin K and lissoclinidine: Novel bioactive alkaloids from the New Zealand ascidian Lissoclinum notti. Tetrahedron 2002, 58, 9779–9783. [Google Scholar] [CrossRef]
- Copp, B.R.; Jompa, J.; Tahir, A.; Ireland, C.M. Styelsamines A—D: New tetracyclic pyridoacridine alkaloids from the Indonesian ascidian Eusynstyela Latericius. J. Org. Chem. 1998, 63, 8024–8026. [Google Scholar] [CrossRef]
- Bry, D.; Banaigs, B.; Long, C.; Bontemps, N. New pyridoacridine alkaloids from the purple morph of the ascidian Cystodytes dellechiajei. Tetrahedron Lett. 2011, 52, 3041–3044. [Google Scholar] [CrossRef]
- Fong, H.K.H.; Copp, B.R. Synthesis, DNA Binding and Antitumor Evaluation of Styelsamine and Cystodytin Analogues. Mar. Drugs 2013, 11, 274–299. [Google Scholar] [CrossRef] [Green Version]
- Charyulu, G.A.; McKee, T.C.; Ireland, C.M. Diplamine, a cytotoxic polyaromatic alkaloid from the tunicate Diplosoma sp. Tetrahedron Lett. 1989, 30, 4201–4202. [Google Scholar] [CrossRef]
- Clement, J.A.; Kitagaki, J.; Yang, Y.; Saucedo, C.J.; O’Keefe, B.R.; Weissman, A.M.; McKee, T.C.; McMahon, J.B. Discovery of new pyridoacridine alkaloids from Lissoclinum c F. Badium that inhibit the ubiquitin ligase activity of Hdm2 and stabilize p53. Bioorg. Med. Chem. 2008, 16, 10022–10028. [Google Scholar] [CrossRef] [Green Version]
- Searle, P.A.; Molinski, T.F. Five new alkaloids from the tropical ascidian, Lissoclinum sp. lissoclinotoxin A is chiral. J. Org. Chem. 1994, 59, 6600–6605. [Google Scholar] [CrossRef]
- Molinski, T.F.; Ireland, C.M. Varamines A and B, new cytotoxic thioalkaloids from Lissoclinum vareau. J. Org. Chem. 1989, 54, 4256–4259. [Google Scholar] [CrossRef]
- Rudi, A.; Benayahu, Y.; Goldberg, I.; Kashmana, Y. Alkaloid metabolites of the marine tunicate: Segoline A, isosegoline A and nor-segoline. Tetrahedron Lett. 1988, 29, 3861–3862. [Google Scholar] [CrossRef]
- Rudi, A.; Kashman, Y. Six new alkaloids from the purple Red Sea tunicate Eudistoma sp. J. Org. Chem. 1989, 54, 5331–5337. [Google Scholar] [CrossRef]
- Einat, M.; Nagler, A.; Lishner, M.; Amiel, A.; Yarkoni, S.; Rudi, A.; Gellerman, G.; Kashman, Y.; Fabian, I. Potent antileukemic activity of the novel agents norsegoline and dibezine. Clin. Cancer Res. 1995, 1, 823–829. [Google Scholar] [PubMed]
- Kim, J.; Pordesimo, E.O.; Toth, S.I.; Schmitz, F.J.; Van Altena, I. Pantherinine, a cytotoxic aromatic alkaloid, and 7-deazainosine from the ascidian Aplidium pantherinum. J. Nat. Prod. 1993, 56, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Handbook of Anticancer Drugs from Marine Origin; Springer: Berlin, Germany, 2015. [Google Scholar]
- Carrol, A.R.; Scheuer, P.J. Kuanoniamines A, B, C, and D: Pentacyclic alkaloids from a tunicate and its prosobranch mollusk predator Chelynotus semperi. J. Org. Chem. 1990, 55, 4426–4431. [Google Scholar] [CrossRef]
- Eder, C.; Schupp, P.; Proksch, P.; Wray, V.; Steube, K.; Muller, C.E.; Frobenius, W.; Herderich, M.; Van Soest, R.W.M. Bioactive pyridoacridine alkaloids from the Micronesian sponge Oceanapia sp. J. Nat. Prod. 1998, 61, 301–305. [Google Scholar] [CrossRef]
- Bontemps, N.; Gattacceca, F.; Long, C.; Thomas, O.P.; Banaigs, B. Additional cytotoxic pyridoacridine alkaloids from the ascidian Cystodytes violatinctus and biogenetic considerations. J. Nat. Prod. 2013, 76, 1801–1805. [Google Scholar] [CrossRef]
- Salomon, C.E.; Faulkner, D.J. Sagitol, a pyridoacridine alkaloid from the sponge Oceanapia sagittaria. Tetrahedron Lett. 1996, 37, 9147–9148. [Google Scholar] [CrossRef]
- Utkina, N.K. Sagitol D, a new thiazole containing pyridoacridine alkaloid from a vietnamese ascidian. Nat. Prod. Commun. 2015, 10, 1547–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.R.; Mohamed, G.A.; Elkhayat, E.S.; Fouad, M.A.; Proksch, P. Sagitol C, a new cytotoxic pyridoacridine alkaloid from the sponge Oceanapia sp. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Gunawardana, G.P.; Koehn, F.E.; Lee, A.Y.; Clardy, J.; He, H.Y.; Faulkner, D.J. Pyridoacridine alkaloids from deep-water marine sponges of the family Pachastrellidae: Structure revision of dercitin and related compounds and correlation with the kuanoniamines. J. Org. Chem. 1992, 57, 1523–1526. [Google Scholar] [CrossRef]
- Gunawardana, G.P.; Kohmoto, S.; Gunasekera, S.P.; McConnell, O.J.; Koehn, F.E. Dercitine, a new biologically active acridine alkaloid from a deep water marine sponge, Dercitus sp. J. Am. Chem. Soc. 1988, 110, 4856–4858. [Google Scholar] [CrossRef]
- Gunawardana, G.P.; Kohmoto, S.; Burres, N.S. New cytotoxic acridine alkaloids from two deep water marine sponges of the family. Tetrahedron Lett. 1989, 30, 4359–4362. [Google Scholar] [CrossRef]
- Burres, N.S.; Sazesh, S.; Gunawardana, G.P.; Clement, J.J. Antitumor activity and nucleic acid binding properties of dercitin, a new acridine alkaloid isolated from a marine Dercitus species sponge. Cancer Res. 1989, 49, 5267–5274. [Google Scholar]
- Gullo, V.P. Discovery of Novel Natural Products with Therapeutic Potential; Newnes: Oxford, UK, 2013. [Google Scholar]
- Torres, Y.R.; Bugni, T.S.; Berlinck, R.G.S.; Ireland, C.M.; Ferreira, A.G.; da Rocha, R.M. Sebastianines A and B, novel biologically active pyridoacridine alkaloids from the Brazilian ascidian Cystodytes dellechiajei. J. Org. Chem. 2002, 67, 5429–5432. [Google Scholar] [CrossRef]
- Plubrukarn, A.; Davidson, B.S. Arnoamines A and B, new cytotoxic pentacyclic pyridoacridine alkaloids from the ascidian Cystodytes sp. J. Org. Chem. 1998, 63, 1657–1659. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Agarwal, S.K.; Gunasekera, S.P.; Schmidt, P.G.; Shoolery, J.N. Amphimedine, new aromatic alkaloid from a pacific sponge, Amphimedon sp. Carbon connectivity determination from natural abundance carbon-13-carbon-13 coupling constants. J. Am. Chem. Soc. 1983, 105, 4835–4836. [Google Scholar] [CrossRef]
- Wei, X.; Bugni, T.S.; Harper, M.K.; Sandoval, I.T.; Manos, E.J.; Swift, J.; Van Wagoner, R.M.; Jones, D.A.; Ireland, C.M. evaluation of pyridoacridine alkaloids in a zebrafish phenotypic assay. Mar. Drugs 2010, 8, 1769–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Guzman, F.S.; Carte, B.; Troupe, N.; Faulkner, D.J.; Harper, M.K.; Concepcion, G.P.; Mangalindan, G.C.; Matsumoto, S.S.; Barrows, L.R.; Ireland, C.M. Neoamphimedine: A new pyridoacridine topoisomerase II inhibitor which catenates DNA. J. Org. Chem. 1999, 64, 1400–1402. [Google Scholar] [CrossRef]
- Tasdemir, D.; Marshall, K.M.; Mangalindan, G.C.; Concepcion, G.P.; Barrows, L.R.; Harper, M.K.; Ireland, C.M. Deoxyamphimedine, a New pyridoacridine alkaloid from two tropical Xestospongia sponges. J. Org. Chem. 2001, 66, 3246–3248. [Google Scholar] [CrossRef]
- Marshall, K.; Barrows, L.R. Biological activities of pyridoacridines. Nat. Prod. Rep. 2004, 21, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Nukoolkarn, V.S.; Saen-Oon, S.; Rungrotmongkol, T.; Hannongbua, S.; Ingkaninan, K.; Suwanborirux, K. Petrosamine, a potent anticholinesterase pyridoacridine alkaloid from a Thai marine sponge Petrosia n. sp. Bioorg. Med. Chem. 2008, 16, 6560–6567. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Ngo, A.; Quinn, R.J.; Redburn, J.; Hooper, J.N.A. Petrosamine B, an Inhibitor of the helicobacte rpylori enzyme aspartyl semialdehyde dehydrogenase from the Australian sponge Oceanapia sp. J. Nat. Prod. 2005, 68, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Davis, R.; Sykes, M.L.; Avery, V.; Carroll, A.; Camp, D.; Quinn, R.J. Antitrypanosomal pyridoacridine alkaloids from the Australian ascidian Polysyncraton echinatum. Tetrahedron Lett. 2010, 51, 2477–2479. [Google Scholar] [CrossRef]
- Lindsay, B.S.; Barrows, L.R.; Copp, B. Structural requirements for biological activity of the marine alkaloid ascididemin. Bioorg. Med. Chem. Lett. 1995, 5, 739–742. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Copp, B.R. Anti-tuberculosis natural products: Synthesis and biological evaluation of pyridoacridine alkaloids related to ascididemin. Tetrahedron 2010, 66, 4977–4986. [Google Scholar] [CrossRef]
- Delfourne, E.; Bastide, J. Marine pyridoacridine alkaloids and synthetic analogues as antitumor agents. Med. Res. Rev. 2002, 23, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; DeGuzman, F.S.; Hossain, M.B.; Van der Helm, D. Cytotoxic aromatic alkaloids from the ascidian Amphicarpa meridiana and Leptoclinides sp.: Meridine and 11-hydroxyascididemin. J. Org. Chem. 1991, 56, 804–808. [Google Scholar] [CrossRef]
- Barnes, E.C.; Said, N.A.B.; Williams, E.; Hooper, J.; Davis, R.A. Ecionines A and B, two new cytotoxic pyridoacridine alkaloids from the Australian marine sponge, Ecionemia geodides. Tetrahedron 2010, 66, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.; Pham, N.B.; Quinn, R.J. Structure determination of pentacyclic pyridoacridine alkaloids from the Australian marine organisms Ancorina geodides and Cnemidocarpa stolonifera. Eur. J. Org. Chem. 2014, 2014, 4805–4816. [Google Scholar] [CrossRef]
- Carroll, A.R.; Cooray, N.M.; Poiner, A.; Scheuer, P.J. A second shermilamine alkaloid from a tunicate Trididemnum sp. J. Org. Chem. 1989, 54, 4231–4232. [Google Scholar] [CrossRef]
- Cooray, N.M.; Scheuer, P.J.; Parkanyi, L.; Clardy, J. Shermilamine A: A pentacyclic alkaloid from a tunicate. J. Org. Chem. 1988, 53, 4619–4620. [Google Scholar] [CrossRef]
- Koren-Goldshlager, G.; Aknin, M.; Gaydou, E.M.; Kashman, Y. Three new alkaloids from the marine tunicate Cystodytes violatinctus. J. Org. Chem. 1998, 63, 4601–4603. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Hwang, J.S.; Glazer, E.; Gut, D.; Kol, M.; Tor, Y. Eilatin Ru(II) complexes display anti-HIV activity and enantiomeric diversity in the binding of RNA. ChemBioChem 2002, 3, 766–771. [Google Scholar] [CrossRef]
- Zeng, C.-M.; Ishibashi, M.; Matsumoto, K.; Nakaike, S.; Kobayashi, J. Two new polycyclic aromatic alkaloids from the Okinawan marine sponge Biemna sp. Tetrahedron 1993, 49, 8337–8342. [Google Scholar] [CrossRef]
- Aoki, S.; Wei, H.; Matsui, K.; Rachmat, R.; Kobayashi, M. Pyridoacridine alkaloids inducing neuronal differentiation in a neuroblastoma cell line, from marine sponge Biemna fortis. Bioorg. Med. Chem. 2003, 11, 1969–1973. [Google Scholar] [CrossRef]














































| Activity | Substituents | |||
|---|---|---|---|---|
| R | R1 | R2 | R3 | |
| anticancer | H | H, OCH3 | H, OCH3 | H, OCH3 |
| antimalarial | H, CH3 | H | H | H |
| antifungal | H, CH3 | H | H | H |
| antibacterial | H, CH3 | H | H | H |
| Activity | Substituent | ||
|---|---|---|---|
| R | R1 | R2 | |
| anticancer | H, COCH3 | SCH3 | H |
| antimicrobial | COCH3 | H, SCH3 | H, SCH3 |
| Alkaloid | Activity | IC50 |
|---|---|---|
| 153 | antiproliferative inhibition of polymerase I stabilization of protein-DNA complexes | 63–150 nM |
| 154 | antiproliferative | 4.8 μM |
| 155 | antiproliferative | 26.7 μM |
| 156 | antiproliferative | 12.0 μM |
| 157 | antiproliferative | 1.9 μM |
| 158 | antiproliferative | 9.9 μM |
| 159 | antiproliferative | 60.0 μM |
| Activity | Substituent | |
|---|---|---|
| R | R1 | |
| AChE inhibitor | H | Br |
| antibacterial (H. pylori) | Br | H |
| inactive | H | H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabowska, G.; Barg, E.; Wójcicka, A. Biological Activity of Naturally Derived Naphthyridines. Molecules 2021, 26, 4324. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144324
Chabowska G, Barg E, Wójcicka A. Biological Activity of Naturally Derived Naphthyridines. Molecules. 2021; 26(14):4324. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144324
Chicago/Turabian StyleChabowska, Gabriela, Ewa Barg, and Anna Wójcicka. 2021. "Biological Activity of Naturally Derived Naphthyridines" Molecules 26, no. 14: 4324. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144324