2. Results and Discussion
We have now studied the hydrolysis–condensation reaction of phenyltrichlorosilane (
1) at different concentrations of HCl (C
HCl) in water-acetone solutions. Then, the thermal self-condensations in “pseudo”-equilibrium conditions (under atmospheric pressure) of four isomeric tetrahydroxy-(tetraphenyl)cyclotetrasiloxanes: all-
cis- (
2),
cis-cis-trans- (
3),
cis-trans-(
4), and all-
trans-(
5) (
Figure 1) have been performed.
All reactions were monitored with APCI-MS and
29Si NMR spectroscopy. The reactions of
1 were conducted at 4 °C with the concentration of HCl between 0.032 and 0.26 mol L
−1. The yields of the products were determined in the interval of 24–570 h. The reaction conditions were selected to be similar to those previously published for three aryltrichlorosilanes [
26], to compare the composition and structure of intermediates and final products.
Figure 2 shows these yields of the products that precipitated from the solution depending on the reaction time. As can be seen from
Figure 2, the rate of the reaction and final yields of the products decreased with the decrease in acidity (See
Note 1 in the Supplementary Materials (SM)).
Figure 3 presents the
29Si NMR spectra of phenylcyclosiloxane compounds obtained at C
HCl = 0.15 and 0.26 mol L
−1, separated from the reaction mixture after the 24 h reaction. Samples of them were dissolved in acetone-
d6 and subjected to
29Si NMR.
A number of signals at −69.0~−71.0 ppm, with one at −69.79 ppm being greatly predominant, were observed for the products obtained at C
HCl = 0.15 and 0.26 mol L
−1, while any signals in the regions of −50.0 and −80.0 ppm were absent. This evidenced that the products had only one OH group at each silicon atom. All of this was valid for compounds from the reaction conducted at C
HCl = 0.055 mol L
−1. The TLC tests of all the products with the toluene:diethyl ether = 4:1 eluent gave a value of R
f = 0.05 that was consistent with previously published data for all-
cis-isomer of (tetrahydroxy)(tetraphenyl)cyclotetrasiloxane (
2) [
27,
28]. The small signals around the intensive one most likely belonged to other isomers of (tetrahydroxy)(tetraphenyl)cyclotetrasiloxane. The calculation of the ratio of the integral intensity of the main signal to the sum of it and the integral intensities of the small signals around it made for spectrum “c” in
Figure 3 showed that the content of isomer
2 in the mixture of isomers ought to be higher than 95%.
It was of interest to compare the results of the hydrolysis-condensation reaction of PhSiCl
3 with those of hydrolysis-condensations of
m-tolylSiCl
3,
m-ClPhSiCL
3, and
α-naphtylSiCl
3 reported in [
26].
For all these compounds, the rates of the reactions and the yields of products increased with the increase in the acidity of the reaction mixtures.
However, there were differences in the types of the products.
Thus, the
29Si NMR spectra of the products (
Figure 3) showed that, in the hydrolysis–condensation reaction of
1, at different C
HCl in the solutions, isomer
2 formed mostly, and other isomers formed in negligible amounts or isomerization of
2 to them occurred to a very small extent, even if the reaction was conducted for 120 h. Compound
2 was obtained in the 73.6% yield (See
Section 3.3.1).
The
29Si NMR spectra in
Figure 3 (the spectra of the precipitates when the reaction was performed at the C
HCl = 0.055 mol L
−1 were similar) were compared with those for the hydrolysis–condensation of
m-tolylSiCl
3 implemented at the C
HCl = 0.054 mol L
−1 and reported in [
26]. In the former case, as was told above, the intensive singlet of
2 at −69.79 ppm was present in the spectra. At the same time, in the latter case, two singlets at −69.98 and −70.49 ppm (these values were more accurately measured than those given in [
26]) were first present in the
29Si NMR spectrum, the upfield singlet being of an even greater intensity than the downfield one. Then the intensity of the upfield signal decreased in time until it completely disappeared, and only the downfield singlet remained in the spectrum. The upfield signal was ascribed to the
cis-
trans-isomer of (tetrahydroxy)(tetra-
m-tolyl)cyclotetrasiloxane (In [
26], the
cis-
trans-isomer was mistakenly named as the
cis-
trans-
cis-isomer) which graded into all
cis-isomer, that gave the downfield signal at −69.98 ppm.
Two singlets in the region of −71.3 to −72.3 ppm (the more accurate measurements gave values −71.55 and −71.78 ppm) were found in the
29Si NMR spectrum of the precipitate from the reaction mixture of the hydrolysis–condensation of
m-ClPhSiCl
3 carried out at C
HCl = 0.032 mol L
−1 for 48 h. They were ascribed to the all
cis- and
cis-
trans-isomers of (tetrahydroxy)(tetra-
m-chlorophenyl)cyclotetrasiloxanes, respectively [
26].
Two singlets at −60.68 and −61.64 ppm were present in the 29Si NMR spectrum of the precipitate from the reaction of the hydrolysis–condensation of α-naphtylSiCl3 conducted at CHCl = 0.15 mol L−1. Most likely, they belonged to isomers of 1,1,3,3-tetrahydroxy(1,3-di-α-naphtyl)disiloxane.
What is the reason for such different steric results for the reactions of
1 and its aryl analogs? Probably, in the last three cases,
trans-isomers of 1,1,3,3-tetrahydroxy(1,3-diaryl)disiloxanes formed as intermediates along with the
cis-isomers. They seem to be more stable than the
cis-ones since the
m-tolyl,
m-ClPh, and
α-naphtyl substituents are more bulky than the Ph one, and the reaction partly occurred through them. The condensation in the case of
1 occurred completely via the formation of the
cis-isomer as an intermediate (
Scheme 1).
As an indirect evidence in support of
Scheme 1, it is necessary to mention that the
trans-isomer of 1,1,3,3-tetrahydroxy-1,3-di-
α-naphthyldisiloxane was obtained in 48% yield by the hydrolysis–condensation reaction of
α-naphtylSiCl
3 carried out at C
HCl = 0.15 mol L
−1 for 190 h. It was characterized by the XRPD method [
26].
Thus, in the case of m-tolylSiCl3, though the analog of 2, all cis-(tetrahydroxy)(tetra-m-tolyl)cyclotetrasiloxane was obtained in the 30.8% yield, the cis-trans-isomer formed in significant amounts in the course of the reaction. It was then graded into all cis-isomer. This was not characteristic of the reactions of 1.
Besides, at a greater acidity, a mixture containing many m-tolylcyclosiloxanes was obtained in the course of the 24 h reaction of m-tolylSiCl3, as was shown by PI APCI-MS.
In the case of m-ClPhSiCL3, two isomers of (tetrahydroxy)(tetra-m-chlorophenyl)cyclotetrasiloxane were obtained by the hydrolysis-codensation reaction of it: the all-cis one in the yield of 19% and the cis-trans-isomer in the yield of 24.9%. Apart from this, the formation of poly-m-chlorophenylsilsesquioxanes in the course of the reaction was displayed by 29Si NMR.
The trans-isomer of 1,1,3,3-tetrahydroxy-1,3-di-α-naphtyldisiloxane was obtained in the 48% yield by the hydrolysis–condensation reaction of α-naphtylSiCl3. Besides, PI APCI-MS showed some other condensation products (e.g., α-naphtyl-T8 and α-naphtyl-T10) to be present in the reaction mixtures.
As it was told above, other isomers apart from
2 were formed in negligible amounts in the course of the hydrolysis-condensation reaction of
1. However, it was earlier reported that
2 underwent isomerization to the other isomers in a dilute acetone solution if hydrochloric acid or methylchlorosilanes were added to the solution [
27,
28,
29].
Recently, the stereoisomerization of all
cis-[
iso-C
4H
9Si(OH)O]
4 in an acetone solution under the action of HCl taken in concentrations from 0.01 M to 5 M was studied. It was found that after 10 min, regardless of the C
HCl, three isomers were formed and the ratio between the four isomers changed insignificantly with the change in C
HCl [
30].
We prepared these other isomers of
2 with the protocol reported in [
28].
The isomers were separated by fractional crystallization and their identification was first made by the R
f values and IR spectra [
27]. According to the
29Si NMR spectral data, the presence of 5% of any isomer
2–
5 in solution could easily be determined [
28]. Thus, we used
29Si NMR for the additional identification of the isomers. With that, it was taken into account that the chemical shifts (δ) for stereoisomers
2–
5 changed depending on the concentration and composition of the mixture, however the differences (Δδ) between three singlets of isomer
2,
4, and
5 were constant, whereas isomer
3 gave three singlets with the ratio of 1:2:1. APCI-MS was also employed for this purpose in the cases of
2 and
3 (see below). Previously, structures of
2 and
5 were confirmed by X-ray powder diffraction and single crystal analysis [
31] and [
10], respectively
Stereoisomers 2–5 were used as the starting compounds for the condensations carried out under “pseudo”-equilibrium conditions (under atmospheric pressure). As it was told above, APCI-MS and 29Si NMR were employed for monitoring the reactions.
As mentioned above, the APCI-MS was also used in the identification of isomers
2 and
3. However, these compounds, as well as
4 and
5, could enter into the self-condensation reaction under conditions of APCI–MS. Because of this, the PI (positive ion mode) and NI (negative ion mode) APCI mass spectra of
2 and
3 were thoroughly analyzed and additional spectra were recorded. It was believed that this would allow us to understand what results corresponded to the reactions in flasks rather than in the mass spectrometer. Samples of
2 and
3 were dissolved in acetonitrile before introduction in the instrument.
A priori, the results could depend on the concentration of
2 or
3 in this solvent. That is why the spectra of
2 were recorded at different dilutions.
Figure 4 shows these mass spectra.
When the concentration of
2 was relatively high, the PI APCI mass spectrum displayed a peak at
m/z 570 due to ion [M
2 + H
2O]
+• (See
Note 2 in the SM), whereas peaks of [M
2]
+• and [M
2 + H]
+ were virtually absent (
Figure 4a). In the spectrum, the maximal ion peak proved to be that of a compound formed owing to the uncompleted condensation of two molecules of
2 with a water molecule added in the mass spectrometer ([(M
2)
2 − 2H
2O) + H
2O]
+• at
m/z 1086). Ion [(M
2)
2 − H
2O) + H
2O]
+• at
m/z 1104 and ions from a dimer formed due to the loss of three water molecules in the course of the condensation (also with the addition of H
2O during the analysis) were also present (See
Note 3 in the SM).
However, when the analyte was significantly diluted with acetonitrile, the self-condensation of
2 was almost suppressed, and the mass spectrum displayed the [M
2 + H
2O]
+• ion peak being predominant (
Figure 4b).
In the NI APCI mass spectrum of the former variant of analyte, ion peaks of anions [M
2 − H]
− at
m/z 551 and [(M
2 − H)M
2]
− or [((M
2)
2 − H
2O) − H + H
2O]
− at
m/z 1103 were present. For the latter (diluted) analyte, the peak of the dimer was absent (
Figure 4c,d, respectively; also see
Note 4 in the SM).
For compound
3, the most abundant ion peak in PI APCI mass spectra turned out to be one of radical cation [M
3 + H
2O]
+•, and ion peaks due to higher molecular mass compounds were more abundant than in the case of
2 (See
Figure S1 in the SM where ions recorded by APCI-MS are specified).
The results obtained showed that, when analyzing the reaction mixtures of the self-condensation of species 2–5 by APCI-MS, if the starting isomer still remained in the mixture, the samples for analysis should sufficiently be diluted to avoid additional reactions occurring immediately inside the mass spectrometer.
Moreover, polyhedral compounds: octaphenylsilsesquioxane (Ph-T
8) and dodecaphenylsilsesquioxane (Ph-T
12), (
6 and
7, respectively) were awaited to be formed as products of condensations of species
2–
5. Though APCI-MS is considered as a rather mild method of ionization, fragmentations of ions could occur, especially as “in source collision induced dissociations”, owing to the construction peculiarity of the instrument [
32,
33]. Thus, it was also interesting to obtain the APCI mass spectra of
6 and
7 and estimate how the fragmentations of ions of these compounds could hinder understanding what products and intermediates formed in the course of the reactions in flasks. Compounds
6 and
7 were obtained by the corresponding protocols described in [
34] and [
14], respectively. Their APCI mass spectra are presented in
Figure 5 and
Figure 6.
Figure 5 and
Figure 6 displayed fragment ions that exist in the PI APCI mass spectra for
6 and in the NI mode for compound
7. As underlined above, this should be taken into account when analyzing the reaction mixtures with these methods.
The spectra also demonstrated both these compounds to be registered in the PI mode (analogously to
2 and
3) as hydrates of their ions (see the last sentence of
Note 2 in the SM) with the molecular ions being absent. Spectrum
6b showed that all of this was valid for compound
7, even in the NI mode.
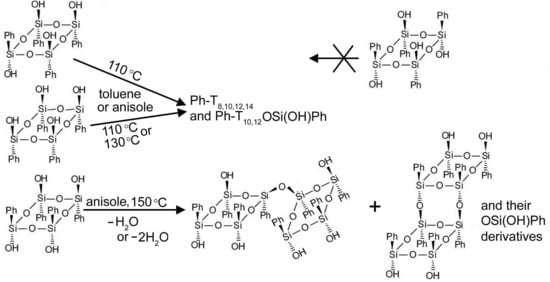
The reaction of condensation of compound
2 was first performed in toluene solution in a quartz flask at 110 °C for 120 min (
Note 5 in the SM). The PI APCI mass spectra of the precipitates from the reaction mixture were taken after 30, 45, and 120 min periods. The spectra of the precipitates obtained after the 45 and 120 min periods are given in
Figure S2 in the SM and
Figure 7a, respectively. They do not contain peaks at
m/z 570, characteristic of the starting compound. This means that they show products of the solution reaction rather than those of the reaction that occurred in the mass spectrometer.
Of interest are groups of the most intensive peaks at
m/z 1566, 1446, 1308, 1291, 1254, and 996 (the peaks of the nominal mass ions) in
Figure 7a. The 1566 group obviously belongs to the hydrate of compound
7 (Ph-T
12), and the lower mass ions are not its fragment ions. This comes from the fact that the fragmentations were not observed in the PI APCI mass spectrum of pure
7 (See
Figure 6a). Moreover, the ms
2 spectrum of the 1568 Da ion supported this. Also, the ions of the 1308 ion group are not the fragment ions of the 1446 one, since the ms
2 spectrum of the 1446 Da ion showed it. Thus, the 1446 and 1308 ion groups correspond to the hydrates of products of the reaction in the flask. The latter is obviously present due to the hydrate of compound
8 (Ph-T
10, see
Figure 7a). The former group can be attributed to the hydrates of species
9, (Ph-T
10OSi(OH)Ph, also see
Figure 7a) and/or the isomeric one where the OSi(OH)Ph group is connected to the polyhedral cage with breakage of a vertical edge rather than one in a pentagon. The ion with the nominal mass 1291 Da could be the protonated molecule of
8, and/or a fragment ion of [M
8 + H
2O]
+• formed via the loss of
•OH from it. However, the ms
2 spectrum of the 1308 Da ion showed the fragmentation producing the 1290 Da ion (the loss of a molecule of water). Thus, the 1291 Da ion appears to be the protonated molecule of the reaction product
8.
One more product was found, compound
6 (Ph-T
8) registered as the molecular radical cation of its hydrate, [M
6 + H
2O]
+•. The 996 Da ion appeared to be its fragment ion formed due to the loss of three water molecules from this radical cation. This found support from the finding that this ion was present in the PI APCI mass spectrum of pure
6 (
Figure 5). A similar process was observed for the 1308 Da ion to give the 1254 Da ion.
Two other products were detected. Their possible structures are depicted in
Figure 7 under numbers
10 (Ph-T
12OSi(OH)Ph) and
11 (Ph-T
14)).
Two comparative experiments were made with the precipitate from the 120 min reaction mixture. Samples of it in acetonitrile were introduced in the mass spectrometer, either water or heavy water (D
2O) being introduced simultaneously.
Figure 7b,c give the PI APCI mass spectra in the region of the molecular ions of the compound
7 hydrates for the H
2O and D
2O cases, respectively. As expected, in the second spectrum, the peak of the ion with the nominal mass of 1566 Da shifted by two units to that of 1568 Da, indicating the addition of a D
2O molecule. An interesting fact was also observed: a peak at
m/z 1584 of the dihydrate was registered at
m/z 1588. This indicated the addition of two water or heavy water molecules to the molecular ion of
7 ([M
7]
+•) in the mass spectrometer (see
Note 6 in the SM). A group of peaks beginning with that at
m/z 1602, belonging to the trihydrate, were found in spectrum 7a. Most likely, the third water molecule added to [M
7]
+• in the mass spectrometer; the abundances of the corresponding peaks in spectrum 7b, however, were too small to make the correct conclusion.
The mass spectra of the solution and the precipitate from the same time reaction mixture were virtually similar. This proved to be so for the reactions of 2–4 carried out under any other conditions employed.
As mentioned before,
Figure S2 in the SM and
Figure 7a present the PI-APCI mass spectra recorded after 45 and 120 min of heating of compound
2 at 110 °C in toluene, respectively. They reflected changes in the reaction mixture in time: a decrease in products of the condensation of two molecules of
2 (peaks of the nominal mass ions at
m/z 996 and 1050) and an increase in those of three and four molecules (peaks at
m/z 1254, 1291, 1308, 1446, 1566, 1584, and 1704, 1824, respectively).
It was previously reported that, when implementing the zone melting of a mixture of isomeric cyclosiloxanes in a molybdenum glass ampoule, a polymerization process was observed. The authors believed this to occur owing to the breakage of the cyclosiloxane rings catalyzed by metal ions present in the glass [
35]. Bearing this in mind, we carried out the reaction of
2 in a molybdenum glass flask in toluene at 110 °C for 120 min. In the PI-APCI mass spectrum of the precipitate (
Figure S3 in the SI), all ion peaks that were found in the corresponding spectrum of the precipitate from the reaction in the quartz flask were present. However, the ion peaks due to product
9 turned out to be significantly greater in abundances. As mentioned above, it probably could be owing to the catalysis by metal ions present in molybdenum glass, however, further thorough experiments are required to prove this.
The
29Si NMR spectrum of this precipitate showed two groups of signals, at −68.0~−71.0 and −76.0~−79.5 ppm. The former contained signals of low intensities belonging to the –OSi(OH)Ph groups. The latter consisted of narrow well-resolved signals at −78.5~−79.5 ppm related to PhSiO
1.5 fragments and also narrow signals at −76.8~−77.9 ppm attributed to the PhSiO
1.5 group in the PhSiO
1.5-PhSi(OH)O-PhSiO
1.5 fragments. Earlier, we observed similar upfield signals at −76.0~−77.5 ppm in the spectra of PPSSO with
Mw = 1300–16000 [
12]. These results were in good accord with those of APCI-MS (See
Figure S3 in the SM).
To increase the temperature at which the reaction of
2 was conducted, it was carried out in anisole as a solvent in a molybdenum glass flask at the temperature of 130 °C. The mass spectra of the precipitates were obtained after 5 and 15 min from the beginning of the reaction. The ion peak due to the starting compound
2 was absent even in the former spectrum. The latter one is given as
Figure S4 in the SM. In this spectrum, all ions of products being in the spectrum of the precipitate from the mixture obtained after the reaction was implemented in toluene, also in a molybdenum glass flask, at 110 °C for 120 min (
Figure S3 in the SM) are present. However, in the spectrum
S3, the ion peaks of the monohydrate of compound
9 with the 1446 Da nominal mass were maximal in abundance, while all other peaks, except those at
m/z 996 and 997, were of minor ones. On the contrary,
Figure S4 shows several peaks to be abundant (of ions with the 996, 1254, 1291, 1308, 1326, 1446, 1464, 1566, 1584, and 1704 Da nominal masses) (see
Figure S4 for the structures of these products and ions.) Two facts are of interest here. The first is the great abundance of peaks of dihydrates in products
7,
8, and
9. Earlier, we showed that two water molecules really added to molecular ions in the mass spectrometer (see
Note 6 in the SM). However, the abundances of the corresponding peaks are rather great, and the reaction time was rather short in this case. Hence, the contribution of ions of the incompletely closed
7,
8, and
9 formed during the reaction in the flask in the abundances of the corresponding ion peaks cannot be excluded.
The second finding here is that the abundances of ion peaks in this spectrum (and hence the amounts of the corresponding products in the precipitate) of the higher molecular mass products
10 and
11 are greater than in the spectrum of
Figure S3. Moreover, ion peaks of the product
12 hydrate and/or its isomer (See
Figure S4 in the SM for the structure of
12) are present in this spectrum, whereas they are absent in
Figure 7 and
Figure S3. Thus, the reaction of
2 went deeper in this case.
Of interest also is a group of peaks at
m/z 996, 997, 998, and 999. Earlier, we attributed this ion to the fragmentation of the [M
6 + H
2O]
+• radical cation with the elimination of three water molecules. However, another fragmentation seems to be possible. All of this is discussed in
Note 7 in the SM.
The 29Si NMR spectrum of the products of this reaction precipitated for 15 min showed two broad signals at −67.0~−71.0 and −76.5~−80.5 ppm, characteristic of the Ph(HO)SiO and PhSiO1.5 fragments, respectively, with the integral intensity ratio of 1:5. This agreed well with the results of the PI APCI mass spectrum.
The thermal self-condensation of compound
3 was performed in “pseudo”-equilibrium conditions (under atmospheric pressure) in a quartz flask with toluene as a solvent. The reaction was carried out at 110 °C, and samples of the precipitates were taken for the PI APCI analyses after 10, 20, 40, 60, and 120 min from the beginning of the reaction. The 120 min spectrum is given in
Figure 8a. Though the products turned out to be the same, as in the case of compound
2, the abundances of their peaks and thus their contents in the precipitates were rather different from those in the case of
2. All spectra did not virtually contain peaks of initial compound
3 (peaks at
m/z 532 and 570 were present on the background level only). However, peak groups beginning with the peak of the ion with the nominal mass of 996 Da was strongly prevailing in abundance in all the spectra, while the abundances of the other peaks in the spectra were significantly less. This indicated that, in contrast to the case of species
2, the main product ought to be of a relatively lower molecular mass. With that, in moving from the 10 min spectrum to the 20 min one, the relative abundances of the peaks due to the higher molecular mass products slightly increased while, further, these abundances changed insignificantly (compare the spectra in
Figure 8a and
Figure S5 in the SM).
Since compound
4 was poorly soluble in toluene, its condensation was performed in other solvents.
Figure 8b shows the PI APCI mass spectrum of the precipitate from the reaction mixture of the condensation of
4 in anisole at 150 °C for 120 min. The picture here was rather different from that of
Figure 8a. Very small abundance peaks of the initial
4 hydrate at
m/z 570 were detected. The main ions proved to be those of two dimers of
4; formed with the loss of 2H
2O and H
2O, both registered as their hydrates with the nominal masses of 1086 and 1104 Da, respectively. Also present in the spectrum were ions of species
13 and
14 (See
Scheme 2), again detected as the hydrates with the nominal masses 1206 and 1224 Da.
The reaction of
4 in acetonitrile went even deeper at a lower temperature.
Figure S6a in the SM gives the PI-APCI mass spectrum of the precipitate obtained after the heating of the reaction mixture in a quartz flask at 75 °C for 120 min. The main peaks turned out to be of [M
13 + H
2O]
+•, [M
8 + 2H
2O]
+•, and [M
9 + 2H
2O]
+• radical cations with the nominal masses 1206, 1326, and 1464 Da, respectively. At that, as it was discussed above, the last two ions, along with contributions from the dihydrates of species
8 and
9 formed during the MS analysis, could contain contributions from the incompletely condensed
8 and
9 from the reaction that occurred in the flask. When the reaction was prolonged up to 240 min, it again went slightly deeper, with peaks of [M
7 + H
2O]
+• radical cation increasing significantly in abundance. Also, noticeable peaks of [M
10 + H
2O]
+• and [M
11 + H
2O]
+• were registered (
Figure S6b).
Attempts to carry out the self-condensation of isomer 5 under “pseudo”-equilibrium conditions using different solvents (toluene, anisole, or ditolylmethane) in molybdenum glass in the temperature range of 80–155 °C for 30 h failed. No OSSO were detected by MS. An abundant peak of starting isomer 5 was present only.