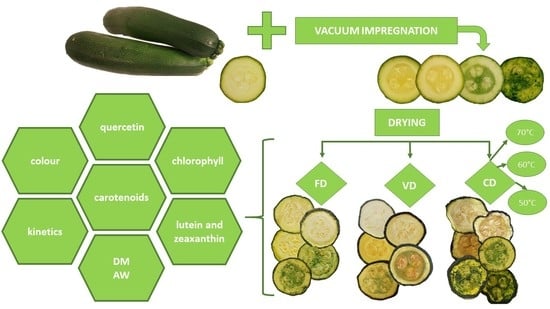
Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dry Matter and Water Activity
2.2. Colour Measurement
2.3. Drying Kinetics
2.4. Chemical Properties
3. Materials and Methods
3.1. Material Preparation
3.2. Impregnating Solutions
3.3. Vacuum Impregnation
3.4. Drying Methods
3.5. Mathematical Modeling
3.6. Dry Matter and Water Activity
3.7. Colour Measurement
3.8. Chemical Properties
3.8.1. Substances and Sample Preparation
3.8.2. Evaluation of Total Carotenoids and Total Chlorophyll
3.8.3. Evaluation of Quercetin and Combined Lutein/Zeaxanthin Contents in Courgette
3.9. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ben-Nun, L. Charakteristics of Zucchini; B.N. Publication House: Beer-Sheva, Isreal, 2019; pp. 8–36. [Google Scholar]
- De Bruin, W.; Rossouw, W.; Korsten, L. Comparison of safe alternative dipping treatments to maintain quality of zucchini. J. Food Qual. 2016, 39, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Alaei, B.; Amiri Chayjan, R. Modelling of nectarin drying under near infrared-vacuum conditions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Chayjan, R.A.; Dibagar, N.; Alaei, B. Drying characteristics of zucchini slices under periodic infrared-microwave vacuum conditions. Heat Mass Transf. 2017, 53, 3473–3485. [Google Scholar] [CrossRef]
- Sokołowska, D.; Kowalczyk, Z. Initial treatment, sublimation drying and storage time of sweet pepper crisps. Physico-chemical quality–Part I. Agric. Eng. 2020, 24, 57–68. [Google Scholar] [CrossRef]
- Sokołowska, D.; Grotkiewicz, K. Initial treatment, sublimation drying and storage time of sweet pepper crisps. Microbiological quality–Part II. Agric. Eng. 2020, 24, 69–78. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.A.; O′Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilmsa, L.C.; Swennen, E.L.R.; Kleinjans, J.C.S.; Bast, A.; Haenen, G.R.M.M. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ (1-42): Relevance to Alzheimer′s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwart, P.; Joashi, A.; Thakur, M. Vacuum Impregnation: A Novel Nondestructive Technique for the Development of Functional Foods. Emerging Technologies in Food Science. In Emerging Technologies in Food Science; Thakur, M., Modi, V., Eds.; Springer: Singapore, 2020; Volume 3, pp. 187–199. [Google Scholar]
- Pasławska, M.; Stępień, B.; Nawirska-Olszańska, A.; Sala, K. Studies on the Effect of Mass Transfer in Vacuum Impregnation on the Bioactive Potential of Apples. Molecules 2019, 24, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponjičan, O.; Sedlar, A.; Findura, P.; Szparaga, A.; Kocira, S. Optimisation of osmotic dehydration of plums. Agric. Eng. 2019, 23, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Alzamora, S.M.; Salvatori, D.; Tapia, M.S.; López-Malo, A.; Welti-Chanes, J.; Fito, P. Novel functional foods from vegetable matrices impregnated with biologically active compounds. J. Food Eng. 2005, 67, 205–214. [Google Scholar] [CrossRef]
- Tylewicz, U.; Lundin, P.; Cocola, L.; Dymek, K.; Rocculi, P.; Svanberg, S.; Dejmek, P.; Galindo, F.G. Gas in scattering media absorption spectroscopy (GASMAS) detected persistent vacuum in apple tissue after vacuum impregnation. Food Biophys. 2012, 7, 28–34. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Pereira, L.M.; Vieira, G.S.; Hubinger, M.D. Mass transfer kinetics of pulsed vacuum osmotic dehydration of guavas. J. Food Eng. 2010, 96, 498–504. [Google Scholar] [CrossRef]
- Junqueira, J.R.D.J.; Corrêa, J.L.G.; Mendonça, K.S.D.; Resende, N.S. Influence of sodium replacement and vacuum pulse on the osmotic dehydration of eggplant slices. Inter. J. Food Sci. Technol. 2017, 41, 10–18. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B.; Islam, M.N. Effect of power ultrasound and pulsed vacuum treatments on the dehydration kinetics, distribution, and status of water in osmotically dehydrated strawberry: A combined NMR and DSC study. Food Bioprocess Technol. 2014, 7, 2782–2792. [Google Scholar] [CrossRef]
- De Lima, M.M.; Tribuzi, G.; de Souza, J.A.R.; de Souza, I.G.; Laurindo, J.B.; Carciofi, B.A.M. Vacuum impregnation and drying of calcium-fortified pineapple snakes. LWT-Food Sci. Technol. 2016, 72, 501–509. [Google Scholar] [CrossRef]
- Song, J.; Meng, L.; Li, D.; Qian, M.; Liu, C. Vacuum impregnation pretreatment with maltose syrup to improve the quality of frozen lotus root. Int. J. Refrig. 2017, 76, 261–270. [Google Scholar] [CrossRef]
- Raponi, F.; Moscetti, R.; Monarca, D.; Colantoni, A.; Massantini, R. Monitoring and Optimization of the Process of Drying Fruits and Vegetables Using Computer Vision: A Review. Sustainability 2017, 9, 2009. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Sagar, V.R.; Suresh Kumar, P. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Orikasa, T.; Ogawa, Y.; Tagwa, A. Vaccum drying characteristics of eggplants. J. Food Eng. 2007, 83, 422–429. [Google Scholar] [CrossRef]
- Dev, S.R.S.; Geetha, P.; Orsat, V.; Gariépy, Y.; Raghavan, G.S.V. Raghavan. Effects of Microwave-Assisted Hot Air Drying and Conventional Hot Air Drying on the Drying Kinetics, Color, Rehydration, and Volatiles of Moringa oleifera. Dry. Technol. 2011, 29, 1452–1458. [Google Scholar] [CrossRef]
- Eissa, H.A.; Bareh, G.F.; Ibrahim, A.A.; Moawad, R.K.; Ali, H.S. The effect of different drying methods on the nutrients and non-nutrients composition of zucchini (green squash) rings. J. Appl. Sci. Res. 2013, 9, 5380–5389. [Google Scholar]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Skarzyńska, K. The application of osmotic dehydration in minimally processed food technology (PL). Postępy Techniki Przetwórstwa Spożywczego 2016, 1, 87–99. [Google Scholar]
- Pasławska, M.; Nawirska-Olszańska, A.; Stępień, B.; Klim, A. The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves. Molecules 2018, 23, 2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawirska-Olszańska, A.; Stępień, B.; Biesiada, A.; Kolniak-Ostek, J.; Oziembłowski, M. Rheological, Chemical and Physical Characteristics of Golden Berry (Physalis peruviana L.) after Convective and Microwave Drying. Foods 2017, 6, 60. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef] [Green Version]
- Pasławska, M.; Sala, K.; Nawirska-Olszańska, A.; Stępień, B.; Pląskowska, E. Effect of Different Drying Techniques on Dehydration Kinetics, Physical Properties, and Chemical Composition of Lemon Thyme. Nat. Prod. Commun. 2020, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Polak, R. Wpływ parametrów sublimacyjnego suszenia na zmianę współrzędnych barwy suszu z liści selera. Acta Sci. Pol. Tech. Agrar. 2008, 7, 9–18. [Google Scholar]
- Ahmed, M.; Akter, M.S.; Eun, J.B. Effect of pretreatments and drying temperatures on sweet potato flour. Inter. J. Food Sci. Technol. 2010, 45, 726–732. [Google Scholar] [CrossRef]
- López-Lluch, D.B.; Cano-Lamadrid, M.; Hernández, F.; Zimmer, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ′Algar′ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods. Molecules 2020, 25, 3643. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Wiktor, A.; Śledź, M.; Witrowska-Rajchert, D. Ultrasound as a Pretreatment Method to Improve Drying Kinetics and Sensory Properties of Dried Apple. J. Food Process Eng. 2016, 39, 256–265. [Google Scholar] [CrossRef]
- Cichowska-Bogusz, J.; Figiel, A.; Carbonell-Barrachina, A.A.; Pasławska, M.; Witrowa-Rajchert, D. Physicochemical Properties of Dried Apple Slices: Impact of Osmo-Dehydration, Sonication, and Drying Methods. Molecules 2020, 25, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, P.; Sharma, H.K. Osmo-convective dehydration kinetics of jackfruit (Artocarpus heterophyllus). J. Saudi Soc. Agric. Sci. 2016, 15, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Li, L. Numerical simulation of mass transfer during the osmotic dehydration of biological tissues. Comput. Mater. Sci. 2006, 35, 75–83. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Effects of Carotenoids on Health: Are All the Same? Results from Clinical Trials. Curr. Pharm. Des. 2017, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Martínez-Valdivieso, D.; Font, R.; Fernández-Bedmar, Z.; Merinas-Amo, T.; Gómez, P.; Alonso-Moraga, Á.; Del Río-Celestino, M. Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects. Nutrients 2017, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and Quantitative Differences in Carotenoid Composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef] [PubMed]
- Samia El-Safy, F. Drying characteristics of loquat slices using different dehydration methods by comparative evaluation. World J. Dairy Food Sci. 2014, 9, 272–284. [Google Scholar]
- Farina, V.; Cinquanta, L.; Vella, F.; Niro, S.; Panfili, G.; Metallo, A.; Cuccurullo, G.; Corona, O. Evolution of carotenoids, sensory profiles and volatile compounds in microwave-dried fruits of three different loquat cultivars (Eriobotrya japonica Lindl.). Plant Foods Hum. Nutr. 2020, 75, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Jałoszyński, K.; Szarycz, M.; Jarosz, B. Wpływ suszenia konwekcyjnego i mikrofalowo-podciśnieniowego na zachowanie związków Aromatycznych w pietruszce naciowej (PL). Inżynieria Rolnicza 2006, 12, 209–215. [Google Scholar]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Rahman, M.S.; Perera, C.O.; Thebaud, C. Desorption isotherm and heat pump drying kinetics of peas. Int. Food Res. J. 1997, 30, 485–491. [Google Scholar] [CrossRef]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K.; Degrmencioglu, A. Mathematical modeling and the determination of some quality parameters of air-dried bay leaves. Biosyst. Eng. 2004, 88, 325–335. [Google Scholar] [CrossRef]
- Midlli, A.; Kucuk, H.; Yapar, Z. A new model for single layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M.; Okyay Menges, H. Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha x piperita L.). Energy Convers. Manag. 2010, 51, 2769–2775. [Google Scholar] [CrossRef]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave drying of parsley: Modeling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Wojdyło, A.; Markowski, J.; Siucińska, K. 1-Methylcyclopropene postharvest treatment and their effect on apple quality during long-term storage time. Eur. Food Res. Technol. 2014, 239, 603–612. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Kolasa, A.; Mościcki, L. Influence of Buckwheat Addition on Physical Properties, Texture and Sensory Characteristics of Extruded Corn Snacks. Pol. J. Food Nutr. Sci. 2013, 63, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]





| Drying Method | Material | Dry Matter [%] | Water Activity [-] |
|---|---|---|---|
| CD 50 | F | 84.92 ± 0.72 d | 0.670 ± 0.037 n |
| N | 80.37 ± 0.32 b | 0.499 ± 0.008 k | |
| O | 75.49 ± 0.33 a | 0.654 ± 0.039 m,n | |
| K | 81.53 ± 0.89 c | 0.644 ± 0.030 m | |
| OK | 80.86 ± 0.61 b | 0.556 ± 0.024 l | |
| CD 60 | F | 90.92 ± 0.59 e | 0.349 ± 0.007 g,h,i |
| N | 94.76 ± 0.80 i,j | 0.355 ± 0.010 g,h,i | |
| O | 91.84 ± 0.28 f | 0.402 ± 0.006 j | |
| K | 90.50 ± 0.90 e | 0.425 ± 0.005 j | |
| OK | 93.56 ± 0.37 h | 0.369 ± 0.006 h,i | |
| CD 70 | F | 91.68 ± 0.35 f | 0.276 ± 0.006 c,d |
| N | 96.76 ± 0.11 k,l | 0.297 ± 0.006 d,e | |
| O | 92.82 ± 0.18 g | 0.335 ± 0.013 f,g | |
| K | 94.38 ± 0.31 i | 0.303 ± 0.008 e | |
| OK | 96.10 ± 0.20 k | 0.330 ± 0.016 f,g | |
| VD | F | 94.65 ± 0.12 i,j | 0.318 ± 0.006 e,f |
| N | 95.23 ± 0.04 j | 0.340 ± 0.009 f,g | |
| O | 96.46 ± 0.16 k | 0.348 ± 0.013 g,h | |
| K | 96.10 ± 0.05 k | 0.317 ± 0.003 e,f | |
| OK | 96.33 ± 0.01 k | 0.375 ± 0.002 i | |
| FD | F | 97.08 ± 0.00 l,m | 0.218 ± 0.006 a |
| N | 95.57 ± 0.17 m,n | 0.255 ± 0.044 b,c | |
| O | 98.12 ± 0.11 n,o | 0.222 ± 0.004 a | |
| K | 97.68 ± 0.07 m,n | 0.239 ± 0.003 a,b | |
| OK | 98.47 ± 0.18 o | 0.255 ± 0.009 b,c |
| Drying Method | Material | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|---|
| - | F | 87.57 ± 0.82 p | −3.28 ± 0.11 k,l | 30.87 ± 0.67 b | 31.04 | - |
| CD 50 | F | 84.94 ± 0.71 n,o | −4.76 ± 0.05 q | 36.95 ± 0.51 h,i,j | 37.96 | 6.79 |
| N | 77.52 ± 0.82 k | −2.92 ± 0.04 m,n | 43.95 ± 0.46 o | 44.05 | 16.50 | |
| O | 60.53 ± 0.57 e,f | −2.69 ± 0.05 o | 38.33 ± 1.01 k,l | 38.42 | 28.60 | |
| K | 52.48 ± 0.48 c | −15.05 ± 0.32 a | 41.57 ± 0.68 n | 44.21 | 38.53 | |
| OK | 62.07 ± 0.70 f | −12.01 ± 0.31 c | 42.88 ± 0.52 o | 44.53 | 29.51 | |
| CD 60 | F | 83.24 ± 1.30 m,n | −3.80 ± 0.08 j | 34.74 ± 0.68 f,g | 34.95 | 5.83 |
| N | 67.61 ±0,47 h | −2.83 ± 0.05 n,o | 38.01 ± 1.01 j,k,l | 38.12 | 21.20 | |
| O | 60.19 ± 0.36 e,f | −2.32 ± 0.04 i | 36.45 ± 0.33 h,i | 36.47 | 28.02 | |
| K | 48.57 ± 0.64 b | −13.31 ± 0.60 b | 39.09 ± 0.67 l | 41.29 | 41.10 | |
| OK | 60.79 ± 1.03 e,f | −10.55 ± 0.49 e | 40.89 ± 0.74 m,n | 42.23 | 29.50 | |
| CD 70 | F | 77.47 ± 0.69 k | −3.14 ± 0.06 l,m | 31.69 ± 0.48 b,c | 31.85 | 10.13 |
| N | 61.77 ± 0.59 f | −2.35 ± 0.03 o | 35.90 ± 0.24 g,h | 35.98 | 26.30 | |
| O | 59.37 ± 1.09 e | −1.25 ± 0.03 p | 34.16 ± 0.67 e,f | 34.18 | 28.46 | |
| K | 42.42 ± 1.45 a | −11.47 ± 0.52 d | 31.89 ± 0.53 c,d | 33.89 | 45.90 | |
| OK | 55.51 ± 1.20 d | −8.65 ± 0.47 g | 38.12 ± 0.42 j,k,l | 39.09 | 33.31 | |
| VD | F | 75.89 ± 0.53 k | −1.58 ± 0.14 p | 24.56 ± 0.82 a | 24.61 | 13.38 |
| N | 71.35 ± 1.00 i | 1.36 ± 0.09 r | 30.68 ± 0.40 b | 30.71 | 13.87 | |
| O | 81.30 ± 0.50 l,m | −1.34 ± 0.06 p | 32.42 ± 0.40 c,d | 32.45 | 6.74 | |
| K | 71.37 ± 2.51 j | −6.61 ± 0.25 h | 39.08 ± 0.70 l | 39.64 | 18.46 | |
| OK | 76.95 ± 1.08 k | −3.55 ± 0.08 j,k,l | 37.64 ± 0.64 i,j,k | 37.81 | 12.60 | |
| FD | F | 80.83 ± 1.06 l | −4.45 ± 0.28 i | 33.40 ± 1.60 d,e | 33.70 | 7.29 |
| N | 85.73 ± 0.82 o,p | −4.70 ± 0.05 i | 32.47 ± 0.74 c,d | 32.81 | 2.82 | |
| O | 75.85 ± 0.70 k | −3.63 ± 0.04 j,k | 34.44 ± 0.54 e,f | 34.63 | 12.26 | |
| K | 59.01 ± 1.60 e | −13.01 ± 0.38 b | 40.32 ± 1.38 m | 42.37 | 31.62 | |
| OK | 65.32 ± 4.60 g | −9.85 ± 0.44 f | 38.92 ± 0.59 l | 40.15 | 24.56 |
| Drying Method | Material | a | b | k | ki | c | R2 | RMSE | χ2 | Ve (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Page Model | ||||||||||
| CD 50 | F | 1.1427 | - | 0.0057 | - | - | 0.9965 | 0.0198 | 0.0004 | 4.4 |
| N | 1.1181 | - | 0.0050 | - | - | 0.9989 | 0.0116 | 0.0001 | 2.5 | |
| O | 1.0274 | - | 0.0072 | - | - | 0.9990 | 0.0099 | 0.0001 | 2.1 | |
| K | 1.1216 | - | 0.0053 | - | - | 0.9969 | 0.0186 | 0.0003 | 4 | |
| OK | 1.0418 | - | 0.0087 | - | - | 0.9985 | 0.0125 | 0.0002 | 2.9 | |
| CD 60 | F | 1.0631 | - | 0.008 | - | - | 0.9967 | 0.0270 | 0.0008 | 5.6 |
| N | 1.1316 | - | 0.0058 | - | - | 0.9932 | 0.0027 | 0.0008 | 5.9 | |
| O | 1.1181 | - | 0.0050 | - | - | 0.9989 | 0.0116 | 0.0001 | 2.5 | |
| K | 1.0631 | - | 0.0080 | - | - | 0.9929 | 0.0297 | 0.0010 | 6.1 | |
| OK | 1.0631 | - | 0.0080 | - | - | 0.9945 | 0.0298 | 0.0010 | 6.7 | |
| CD 70 | F | 1.0873 | - | 0.0080 | - | - | 0.9955 | 0.0204 | 0.0005 | 4.3 |
| N | 1.0435 | - | 0.0091 | - | - | 0.9966 | 0.0182 | 0.0004 | 4 | |
| O | 1.1335 | - | 0.0065 | - | - | 0.9976 | 0.0151 | 0.0002 | 3.1 | |
| K | 1.0633 | - | 0.0089 | - | - | 0.9973 | 0.0155 | 0.0003 | 3.3 | |
| OK | 1.0221 | - | 0.0101 | - | - | 0.9956 | 0.0203 | 0.0004 | 4.5 | |
| Henderson and Pabis Model | ||||||||||
| CD 50 | F | 1.0237 | - | 0.0110 | - | - | 0.9942 | 0.0272 | 0.0008 | 6.3 |
| N | 1.0242 | - | 0.0090 | - | - | 0.9968 | 0.0246 | 0.0005 | 4.4 | |
| O | 1.0020 | - | 0.0082 | - | - | 0.9989 | 0.0110 | 0.0001 | 2.3 | |
| K | 1.0204 | - | 0.0095 | - | - | 0.9945 | 0.0260 | 0.0008 | 5.6 | |
| OK | 1.0040 | - | 0.0106 | - | - | 0.9983 | 0.0142 | 0.0002 | 3.3 | |
| CD 60 | F | 1.0426 | - | 0.0118 | - | - | 0.9915 | 0.0326 | 0.0012 | 7.1 |
| N | 1.0211 | - | 0.0107 | - | - | 0.9904 | 0.0338 | 0.0013 | 7.4 | |
| O | 1.0242 | - | 0.0090 | - | - | 0.9968 | 0.0205 | 0.0005 | 4.4 | |
| K | 1.0187 | - | 0.0101 | - | - | 0.9942 | 0.0257 | 0.0007 | 5.3 | |
| OK | 1.0252 | - | 0.0113 | - | - | 0.9911 | 0.0330 | 0.0012 | 7.4 | |
| CD 70 | F | 1.0135 | - | 0.0118 | - | - | 0.9941 | 0.0245 | 0.0007 | 5.1 |
| N | 1.0026 | - | 0.0111 | - | - | 0.9964 | 0.0197 | 0.0004 | 4.3 | |
| O | 1.0291 | - | 0.0120 | - | - | 0.9947 | 0.0238 | 0.0006 | 4.9 | |
| K | 1.0095 | - | 0.0118 | - | - | 0.9965 | 0.0184 | 0.0004 | 3.9 | |
| OK | 0.9937 | - | 0.0111 | - | - | 0.9958 | 0.0205 | 0.0005 | 4.5 | |
| Logistical Model | ||||||||||
| CD 50 | F | 0.8217 | 1.7679 | 0.0164 | - | 0.9986 | 0.0124 | 0.0001 | 2.9 | |
| N | 1.1789 | 2.1448 | 0.0123 | - | 0.9996 | 0.0068 | 0.0000 | 1.4 | ||
| O | 3.9942 | 4.9340 | 0.0093 | - | 0.9993 | 0.0085 | 0.0000 | 1.8 | ||
| K | 0.9293 | 1.8766 | 0.0138 | - | 0.9985 | 0.0128 | 0.0001 | 2.7 | ||
| OK | 2.4868 | 3.4224 | 0.0126 | - | 0.9990 | 0.0100 | 0.0001 | 2.4 | ||
| CD 60 | F | 0.5939 | 1.5568 | 0.0191 | - | 0.9992 | 0.0092 | 0.0000 | 2 | |
| N | 0.7680 | 1.7039 | 0.0162 | - | 0.9950 | 0.0226 | 0.0008 | 5.8 | ||
| O | 1.1789 | 2.1448 | 0.0123 | - | 0.9996 | 0.0067 | 0.0000 | 1.4 | ||
| K | 1.0250 | 1.9725 | 0.0142 | - | 0.9981 | 0.0141 | 0.0002 | 2.9 | ||
| OK | 0.7368 | 1.6792 | 0.0172 | - | 0.9964 | 0.0197 | 0.0004 | 4.5 | ||
| CD 70 | F | 1.3396 | 2.2843 | 0.0158 | - | 0.9969 | 0.0170 | 0.0004 | 3.6 | |
| N | 2.0664 | 2.9931 | 0.0136 | - | 0.9975 | 0.0154 | 0.0003 | 3.4 | ||
| O | 1.3208 | 2.2983 | 0.0016 | - | 0.9990 | 0.0092 | 0.0000 | 1.9 | ||
| K | 1.9187 | 2.8664 | 0.0147 | - | 0.9981 | 0.0130 | 0.0002 | 2.7 | ||
| OK | 2.2933 | 3.1940 | 0.0134 | - | 0.9967 | 0.0173 | 0.0003 | 3.8 | ||
| Two-term Model | ||||||||||
| CD 50 | F | 0.3334 | 0.6903 | 0.0110 | 0.0110 | - | 0.9942 | 0.0272 | 0.0008 | 6.3 |
| N | 0.3430 | 0.6812 | 0.0090 | 0.0090 | - | 0.9968 | 0.0205 | 0.0005 | 4.4 | |
| O | 0.0072 | 0.6648 | 0.0082 | 0.0082 | - | 0.9989 | 0.0110 | 0.0001 | 2.3 | |
| K | 0.3307 | 0.6896 | 0.0095 | 0.0095 | - | 0.9945 | 0.0260 | 0.0008 | 5.6 | |
| OK | 0.3254 | 0.6786 | 0.0106 | 0.0106 | - | 0.9983 | 0.0114 | 0.0002 | 3.3 | |
| CD 60 | F | 0.3336 | 0.7090 | 0.0118 | 0.0118 | - | 0.9915 | 0.0326 | 0.0013 | 7.1 |
| N | 0.3286 | 0.6926 | 0.0108 | 0.0108 | - | 0.9891 | 0.0350 | 0.0014 | 7.4 | |
| O | 0.3430 | 0.6812 | 0.0090 | 0.0090 | - | 0.9968 | 0.0205 | 0.0005 | 4.4 | |
| K | 0.3290 | 0.6896 | 0.0101 | 0.0101 | - | 0.9942 | 0.0275 | 0.0008 | 5.3 | |
| OK | 0.3291 | 0.6961 | 0.0113 | 0.0113 | - | 0.9911 | 0.0330 | 0.0013 | 7.4 | |
| CD 70 | F | 0.3279 | 0.6856 | 0.0118 | 0.0118 | - | 0.9941 | 0.0245 | 0.0007 | 5.1 |
| N | 0.3404 | 0.6622 | 0.0111 | 0.0111 | - | 0.9964 | 0.0197 | 0.0005 | 4.3 | |
| O | 0.3301 | 0.6990 | 0.0120 | 0.0120 | - | 0.9947 | 0.0238 | 0.0007 | 4.9 | |
| K | 0.3274 | 0.6822 | 0.0118 | 0.0118 | - | 0.9965 | 0.0184 | 0.0004 | 3.9 | |
| OK | 0.3233 | 0.6704 | 0.0111 | 0.0111 | - | 0.9958 | 0.0205 | 0.0005 | 4.5 | |
| Newton Model | ||||||||||
| CD 50 | F | - | - | 0.0107 | - | - | 0.9952 | 0.0288 | 0.0009 | 6.6 |
| N | - | - | 0.0087 | - | - | 0.9978 | 0.0228 | 0.0006 | 4.9 | |
| O | - | - | 0.0082 | - | - | 0.9990 | 0.0110 | 0.0001 | 2.3 | |
| K | - | - | 0.0093 | - | - | 0.9955 | 0.0273 | 0.0008 | 5.8 | |
| OK | - | - | 0.0105 | - | - | 0.9984 | 0.0143 | 0.0002 | 3.4 | |
| CD 60 | F | - | - | 0.0112 | - | - | 0.9937 | 0.0368 | 0.0015 | 8.1 |
| S | - | - | 0.0104 | - | - | 0.9918 | 0.0349 | 0.0014 | 7.6 | |
| O | - | - | 0.0870 | - | - | 0.9978 | 0.0223 | 0.0006 | 4.9 | |
| K | - | - | 0.0098 | - | - | 0.9953 | 0.0269 | 0.0008 | 5.5 | |
| OK | - | - | 0.0109 | - | - | 0.9924 | 0.0345 | 0.0013 | 7.8 | |
| CD 70 | F | - | - | 0.0116 | - | - | 0.9948 | 0.0251 | 0.0007 | 5.3 |
| N | - | - | 0.0111 | - | - | 0.9965 | 0.0020 | 0.0004 | 4.3 | |
| O | - | - | 0.0115 | - | - | 0.9962 | 0.0266 | 0.0008 | 5.5 | |
| K | - | - | 0.0117 | - | - | 0.9970 | 0.0189 | 0.0004 | 3.9 | |
| OK | - | - | 0.0112 | - | - | 0.9956 | 0.0206 | 0.0005 | 4.5 | |
| Midilli et al., Model | ||||||||||
| CD 50 | F | 0.9615 | 0 | 0.0037 | - | 1.2267 | 0.9978 | 0.0155 | 0.0002 | 3.6 |
| N | 0.9777 | 0 | 0.004 | - | 1.1627 | 0.9992 | 0.0092 | 0.0000 | 2 | |
| O | 0.9761 | 0 | 0.0053 | - | 1.0867 | 0.9991 | 0.0095 | 0.0001 | 2 | |
| K | 0.9641 | 0 | 0.0035 | - | 1.2046 | 0.9978 | 0.0154 | 0.0003 | 3.3 | |
| OK | 0.9787 | 0 | 0.0071 | - | 1.0822 | 0.9988 | 0.0117 | 0.0001 | 2.6 | |
| CD 60 | F | 0.9641 | 0 | 0.0028 | - | 1.3025 | 0.9985 | 0.0128 | 0.0002 | 2.8 |
| N | 0.9555 | 0 | 0.0034 | - | 1.2344 | 0.9946 | 0.024 | 0.0007 | 5.2 | |
| O | 0.9778 | 0 | 0.0040 | - | 1.1627 | 0.9993 | 0.0093 | 0.0000 | 2 | |
| K | 0.9662 | 0 | 0.0040 | - | 1.1891 | 0.9973 | 0.0165 | 0.0003 | 3.4 | |
| OK | 0.9539 | 0 | 0.0031 | - | 1.2725 | 0.9952 | 0.0225 | 0.0006 | 5.1 | |
| CD 70 | F | 0.9707 | 0 | 0.0058 | - | 1.1491 | 0.9962 | 0.0187 | 0.0004 | 3.9 |
| N | 0.9739 | 0 | 0.0070 | - | 1.0944 | 0.9971 | 0.0168 | 0.0003 | 3.7 | |
| O | 0.9787 | 0 | 0.0051 | - | 1.1794 | 0.9980 | 0.0137 | 0.0002 | 2.8 | |
| K | 0.9519 | 0 | 0.0044 | - | 1.2085 | 0.9964 | 0.0173 | 0.0004 | 3.6 | |
| OK | 0.9231 | 0 | 0.0030 | - | 1.2758 | 0.9927 | 0.0255 | 0.0255 | 5.6 | |
| Drying Method | Material | Chemical Composition (μg g d.m.−1) | |||
|---|---|---|---|---|---|
| Quercetin | Total Carotenoids | Total Chlorophylls | Lutein and Zeaxanthin | ||
| Not dried | F | 1.82 ± 0.04 a | 298.87 ± 16.01 a | 59.21 ± 4.61 a | 184.91 ± 8.26 a |
| N | ND | 279.44 ± 9.48 a | 39.02 ± 5.76 b | 166.42 ± 9.11 a | |
| O | 51.00 ± 2.63 b | 290.99 ± 18.33 a | 52.07 ± 5.25 a | 188.00 ± 10.87 a | |
| K | 1.90 ± 0.30 a | 349.49 ± 3.51 b | 89.83 ± 7.24 d | 218.44 ± 16.23 b | |
| OK | 11.00 ± 0.39 c | 329.99 ± 16.95 b | 83.27 ± 7.74 d | 203.11 ± 10.00 b | |
| CD 50 | F | ND | 28.36 ± 6.11 c | 11.30 ± 3.36 e | 12.54 ± 0.74 g |
| N | ND | 103.73 ± 12.74 d | 25.81 ± 3.64 c | 52.60 ± 12.32 f | |
| O | 0.82 ± 0.22 d | 110.80 ± 20.61 d | 15.49 ± 2.70 e | 53.49 ± 5.50 f | |
| K | ND | 140.19 ± 25.14 d,e | 63.49 ± 12.23 a | 115.54 ± 20.18 d | |
| OK | 2.97 ± 0.72 a | 97.65 ± 11.76 d | 27.24 ± 2.77 c | 90.20 ± 9.66 e | |
| CD 60 | F | ND | 194.96 ± 34.52 e | 29.56 ± 5.86 c | 165.32 ± 18.86 a |
| N | ND | 132.61 ± 12.61 d,e | 18.24 ± 1.77 e | 100.80 ± 6.94 d | |
| O | 20.38 ± 1.95 e | 124.38 ± 28.91 d,e | 26.55 ± 4.65 c | 135.06 ± 15.60 c | |
| K | ND | 346.85 ± 7.65 d,e | 85.96 ± 18.97 d | 201.82 ± 25.56 b | |
| OK | 8.04 ± 2.37 c | 184.00 ± 16.46 e | 31.68 ± 3.86 b | 123.95 ± 5.64 c | |
| CD 70 | F | ND | 186.76 ± 36.73 e | 28.51 ± 6.31 c | 125.49 ± 17.90 c |
| N | ND | 214.09 ± 14.23 f | 30.72 ± 2.91 b | 157.58 ± 6.28 c | |
| O | 50.94 ± 9.00 b | 152.83 ± 20.46 e | 21.88 ± 3.29 c | 135.31 ± 3.86 c | |
| K | 1.85 ± 0.10 a | 289.35 ± 27.42 a | 67.34 ± 11.59 a | 216.42 ± 25.14 b | |
| OK | 5.58 ± 1.05 a,c | 157.17 ± 4.05 e | 24.12 ± 0.57 c | 129.17 ± 2.93 c | |
| VD | F | 0.75 ± 0.08 d | 174.10 ± 13.63 e,f | 25.84 ± 3.05 c | 138.26 ± 5.81 c |
| N | ND | 134.15 ± 13.54 d,f | 29.93 ± 3.97 c | 105.63 ± 14.12 d | |
| O | 11.80 ± 0.80 c | 151.82 ± 6.11 e | 23.81 ± 0.36 c | 116.69 ± 6.23 d | |
| K | ND | 161.99 ± 21.90 e | 33.18 ± 2.35 b | 140.61 ± 22.64 c | |
| OK | 10.54 ± 0.50 c | 266.71 ± 28.81 a | 52.27 ± 6.86 a | 177.99 ± 22.67 a | |
| FD | F | ND | 266.55 ± 48.67 a | 53.84 ± 9.97 a | 180.94 ± 18.50 a |
| N | ND | 158.66 ± 45.13 e | 34.46 ± 11.12 b | 134.63 ± 39.24 c | |
| O | 41.84 ± 7.22 b | 276.04 ± 43.72 a | 50.43 ± 12.00 a | 162.69 ± 6.32 a | |
| K | 0.86 ± 0.51 d | 267.38 ± 12.86 a | 75.34 ± 4.69 d | 185.89 ± 6.39 a | |
| OK | 7.44 ± 1.50 a,c | 248.78 ± 18.48 a | 48.53 ± 3.76 a | 161.32 ± 12.68 a | |
| Symbol | Infiltration Solutions | °Bx |
|---|---|---|
| N | 3% sodium chloride solution (NaCl) | 4.2 |
| K | Freshly squeezed kale juice + 3% NaCl | 11.5 |
| O | Freshly squeezed onion juice + 3% NaCl | 10.5 |
| KO | Freshly squeezed onion juice and kale juice (50:50) + 2% NaCl | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154597
Kręcisz M, Stępień B, Pasławska M, Popłoński J, Dulak K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules. 2021; 26(15):4597. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154597
Chicago/Turabian StyleKręcisz, Magdalena, Bogdan Stępień, Marta Pasławska, Jarosław Popłoński, and Kinga Dulak. 2021. "Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods" Molecules 26, no. 15: 4597. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154597