Quantitative Determination and Environmental Risk Assessment of 102 Chemicals of Emerging Concern in Wastewater-Impacted Rivers Using Rapid Direct-Injection Liquid Chromatography—Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
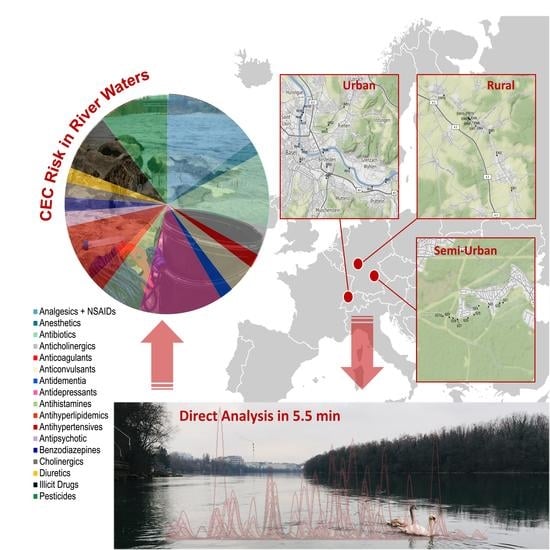
2.2. Sampling Locations and Procedures
2.3. Instrumentation
2.4. Analytical Method Performance Assessment
2.5. Sample Preparation and Quantification Procedures
2.6. Environmental Risk Assessment Procedures
3. Results and Discussion
3.1. Direct-Injection LC-MS/MS Throughput and Sample Volume
3.2. Method Performance Assessment
3.3. Occurrence of Emerging Contaminants in River Water
3.3.1. Campaign 1: R. Schwarzach, Schwarzenbruck District, Germany
3.3.2. Campaign 2: R. Emsbach, Selters, Germany
3.3.3. Campaign 3: R. Rhine, Birsig, Birs, and Wiese at Basel, Switzerland
3.4. Environmental Risk Assessment at High Spatial Resolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability:Samples of the compounds are not available from the authors. |
References
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Environment Programme. Making Peace with Nature: A Scientific Blueprint to Tackle the Climate, Biodiversity and Pollution Emergencies; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Galani, A.; Alygizakis, N.; Aalizadeh, R.; Kastritis, E.; Dimopoulos, M.-A.; Thomaidis, N.S. Patterns of pharmaceuticals use during the first wave of COVID-19 pandemic in Athens, Greece as revealed by wastewater-based epidemiology. Sci. Total Environ. 2021, 798, 149014. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barceló, D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A 2007, 1152, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.C.; Ferguson, P.L. Development of a sensitive direct injection LC-MS/MS method for the detection of glyphosate and aminomethylphosphonic acid (AMPA) in hard waters. Anal. Bioanal. Chem. 2021, 413, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.K.; Chadha, M.; Rapp-Wright, H.; Mills, G.A.; Fones, G.R.; Gravell, A.; Stürzenbaum, S.; Cowan, D.A.; Neep, D.J.; Barron, L.P. Rapid direct analysis of river water and machine learning assisted suspect screening of emerging contaminants in passive sampler extracts. Anal. Methods 2021, 13, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Rapp-Wright, H.; Egli, M.; Hartmann, A.; Steele, J.C.; Sosa-Hernández, J.E.; Melchor-Martínez, E.M.; Jacobs, M.; White, B.; Regan, F.; et al. High-throughput multi-residue quantification of contaminants of emerging concern in wastewaters enabled using direct injection liquid chromatography-tandem mass spectrometry. J. Hazard. Mater. 2020, 398, 122933. [Google Scholar] [CrossRef] [PubMed]
- Askeland, M.; Clarke, B.; Paz-Ferreiro, J. A serial PFASs sorption technique coupled with adapted high volume direct aqueous injection LCMS method. MethodsX 2020, 7, 100886. [Google Scholar] [CrossRef]
- Mosekiemang, T.T.; Stander, M.A.; de Villiers, A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography-tandem mass spectrometry. Chemosphere 2019, 220, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, R.J.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Sánchez-Pérez, J.A.; Malato, S.; Agüera, A. Fast determination of pesticides and other contaminants of emerging concern in treated wastewater using direct injection coupled to highly sensitive ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1507, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Parada, A.; Gómez-Ramos Mdel, M.; Martínez Bueno, M.J.; Uclés, S.; Uclés, A.; Fernández-Alba, A.R. Analytical improvements of hybrid LC-MS/MS techniques for the efficient evaluation of emerging contaminants in river waters: A case study of the Henares River (Madrid, Spain). Environ. Sci. Pollut. Res. Int. 2012, 19, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Busetti, F.; Backe, W.J.; Bendixen, N.; Maier, U.; Place, B.; Giger, W.; Field, J.A. Trace analysis of environmental matrices by large-volume injection and liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlong, E.T.; Noriega, M.C.; Kanagy, C.J.; Kanagy, L.K.; Coffey, L.J.; Burkhardt, M.R. Determination of Human-Use Pharmaceuticals in Filtered Water by Direct Aqueous Injection: High-Performance Liquid Chromatography/Tandem Mass Spectrometry; Techniques and Methods 5-B10; U.S. Geological Survey: Reston, VA, USA, 2014; p. 60.
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of Pharmaceuticals and Personal Care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Hermes, N.; Jewell, K.S.; Wick, A.; Ternes, T.A. Quantification of more than 150 micropollutants including transformation products in aqueous samples by liquid chromatography-tandem mass spectrometry using scheduled multiple reaction monitoring. J. Chromatogr. A 2018, 1531, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Martínez Bueno, M.J.; Uclés, S.; Hernando, M.D.; Fernández-Alba, A.R. Development of a solvent-free method for the simultaneous identification/quantification of drugs of abuse and their metabolites in environmental water by LC–MS/MS. Talanta 2011, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, V.; Helmus, R.; de Voogt, P. Direct injection analysis of polar micropollutants in natural drinking water sources with biphenyl liquid chromatography coupled to high-resolution time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1569, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Couchman, L.; Fisher, D.S.; Subramaniam, K.; Handley, S.A.; Boughtflower, R.J.; Benton, C.M.; Flanagan, R.J. Ultra-fast LC–MS/MS in therapeutic drug monitoring: Quantification of clozapine and norclozapine in human plasma. Drug Test. Anal. 2018, 10, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Critical evaluation of methodology commonly used in sample collection, storage and preparation for the analysis of pharmaceuticals and illicit drugs in surface water and wastewater by solid phase extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 8036–8059. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Choi, P.M.; Thompson, J.; Reeks, T.; Verhagen, R.; Tscharke, B.J.; O’Malley, E.; Shimko, K.M.; Guo, X.; Thomas, K.V.; et al. Systematic Evaluation of the In-Sample Stability of Selected Pharmaceuticals, Illicit Drugs, and Their Metabolites in Wastewater. Environ. Sci. Technol. 2021, 55, 7418–7429. [Google Scholar] [CrossRef]
- Daten & Fakten: Unser Haus in Zahlen. Available online: https://www.sana.de/rummelsberg/ueber-uns/zahlen-fakten (accessed on 8 March 2020).
- Anlagen/Standorte. Available online: https://www.kbv-badcamberg.de/abwasser/anlagen--standorte.html (accessed on 20 January 2020).
- Gesundwerden Inmitten der Taunuslandschaft. Available online: https://www.medicalpark.de/de/Kliniken_und_Zentren/Bad_Camberg.html (accessed on 27 March 2020).
- MEDIAN Hohenfeld-Klinik Bad Camberg. Available online: https://www.median-kliniken.de/de/median-hohenfeld-klinik-bad-camberg/behandlungsgebiete/psychosomatik/ (accessed on 27 March 2020).
- ARA Basel. Available online: https://www.prorheno.ch/anlagen/ara-basel (accessed on 1 August 2021).
- ARA Chemie. Available online: https://www.prorheno.ch/anlagen/ara-chemie (accessed on 1 August 2021).
- Anlagen Birs- und Birsigtal. Available online: https://www.baselland.ch/politik-und-behorden/direktionen/bau-und-umweltschutzdirektion/industrielle-betriebe/abwasseranlagen/anlagen-birs-und-birsigtal (accessed on 1 August 2021).
- ARA Birsig, Therwil. Available online: https://www.baselland.ch/politik-und-behorden/direktionen/bau-und-umweltschutzdirektion/industrielle-betriebe/abwasseranlagen/anlagen-birs-und-birsigtal/downloads/ara-birsig-therwil.pdf/@@download/file/ARA%20Birsig%20-%20Therwil.pdf (accessed on 1 August 2021).
- ARA Birs, Birsfelden. Available online: https://www.baselland.ch/politik-und-behorden/direktionen/bau-und-umweltschutzdirektion/industrielle-betriebe/abwasseranlagen/anlagen-birs-und-birsigtal/downloads/ara-birs-birsfelden.pdf/@@download/file/ARA%20Birs%20-%20Birsfelden.pdf (accessed on 1 August 2021).
- ARA Rhein. Available online: https://www.ararhein.ch/ (accessed on 1 August 2021).
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Seitz, W.; Winzenbacher, R. A survey on trace organic chemicals in a German water protection area and the proposal of relevant indicators for anthropogenic influences. Environ. Monit. Assess. 2017, 189, 244. [Google Scholar] [CrossRef] [PubMed]
- Commission Decision 2002/657/EC of 12 August implementing Council Directive 96/23/EC concerning performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, 50, 8–36.
- 37. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/ EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No. 1488/94 on Risk Assessment for Existing Substances. Part II. Eur. Chem. Bur. 2003, EUR 20418 EN/2, 7–179.
- Palma, P.; Köck-Schulmeyer, M.; Alvarenga, P.; Ledo, L.; Barbosa, I.R.; López de Alda, M.; Barceló, D. Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci. Total Environ. 2014, 488–489, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Vestel, J.; Caldwell, D.J.; Constantine, L.; D’Aco, V.J.; Davidson, T.; Dolan, D.G.; Millard, S.P.; Murray-Smith, R.; Parke, N.J.; Ryan, J.J.; et al. Use of acute and chronic ecotoxicity data in environmental risk assessment of pharmaceuticals. Environ. Toxicol. Chem. 2016, 35, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Aalizadeh, R.; von der Ohe, P.C.; Thomaidis, N.S. Prediction of acute toxicity of emerging contaminants on the water flea Daphnia magna by Ant Colony Optimization–Support Vector Machine QSTR models. Environ. Sci. Process. Impacts 2017, 19, 438–448. [Google Scholar] [CrossRef]
- NORMAN Ecotoxicology Database—Lowest PNECs (Verified). Available online: https://www.norman-network.com/nds/ecotox/lowestPnecsIndex.php (accessed on 2 July 2021).
- Borrull, J.; Colom, A.; Fabregas, J.; Pocurull, E.; Borrull, F. A simple, fast method for the analysis of 20 contaminants of emerging concern in river water using large-volume direct injection liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W. A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Appl. Sci. 2019, 9, 1368. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.; Hernández-Bastida, J.; Cazaña, G.; Pérez-Lucas, G.; Fenoll, J. Assessment of the Leaching Potential of 12 Substituted Phenylurea Herbicides in Two Agricultural Soils under Laboratory Conditions. J. Agric. Food Chem. 2012, 60, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Ng, K.T.; Lamphiere, A.; Cameron, T.C.; Bury, N.R.; Barron, L.P. Multicompartment and cross-species monitoring of contaminants of emerging concern in an estuarine habitat. Environ. Pollut. 2021, 270, 116300. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Ng, K.T.; Bury, S.T.; Bury, S.E.; Bury, N.R.; Barron, L.P. Biomonitoring of pesticides, pharmaceuticals and illicit drugs in a freshwater invertebrate to estimate toxic or effect pressure. Environ. Int. 2019, 129, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Water Quality Monitoring Data GC-MS and LC-MS Semi-Quantitative Screen. Available online: https://environment.data.gov.uk/portalstg/home/item.html?id=b76f059c3f294678840a2590f61f7e59 (accessed on 2 July 2021).
- Rúa-Gómez, P.C.; Püttmann, W. Impact of wastewater treatment plant discharge of lidocaine, tramadol, venlafaxine and their metabolites on the quality of surface waters and groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- ICPR—International Commission for the Protection of the Rhine. Available online: https://www.iksr.org/en/ (accessed on 31 July 2021).
- River Rhine Commended for River Basin Management. Available online: https://www.eea.europa.eu/highlights/river-rhine-commended-for-river (accessed on 1 August 2021).
- Long-Term Annual Average Concentrations (Weil am Rhein). Available online: http://iksr.bafg.de/iksr/lj_auswahl.asp?S=3 (accessed on 31 July 2021).
- Stülten, D.; Zühlke, S.; Lamshöft, M.; Spiteller, M. Occurrence of diclofenac and selected metabolites in sewage effluents. Sci. Total Environ. 2008, 405, 310–316. [Google Scholar] [CrossRef]
- Letzel, M.; Metzner, G.; Letzel, T. Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input. Environ. Int. 2009, 35, 363–368. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Quintana, J.B.; Carpinteiro, J.; Rodríguez, I.; Lorenzo, R.A.; Carro, A.M.; Cela, R. Determination of natural and synthetic estrogens in water by gas chromatography with mass spectrometric detection. J. Chromatogr. A 2004, 1024, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M.; et al. Status of hormones and painkillers in wastewater effluents across several European states—considerations for the EU watch list concerning estradiols and diclofenac. Environ. Sci. Pollut. Res. 2016, 23, 12835–12866. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Reich, M.; Beier, S.; Behrendt, J.; Gulyas, H.; Otterpohl, R. Measured and predicted environmental concentrations of carbamazepine, diclofenac, and metoprolol in small and medium rivers in northern Germany. Environ. Monit. Assess. 2016, 188, 487. [Google Scholar] [CrossRef] [PubMed]






| Analyte | Linearity a | Peak Area Imprecision (RSD%), n = 6 b | Matrix Effect (%), n = 6 c | LLOD d | LLOQ d | ||
|---|---|---|---|---|---|---|---|
| R2 (N > 5) | 250 ng/L | 2500 ng/L | 250 ng/L | 2500 ng/L | ng/L | ng/L | |
| Pharmaceuticals (n = 66) | |||||||
| mean | 0.9930 | 12 | 5 | +42 | +17 | 4 | 11 |
| standard deviation | ±0.0115 | ±18 | ±3 | ±182 | ±58 | ±7 | ±22 |
| median | 0.9967 | 6 | 4 | +5 | +5 | 2 | 5 |
| Illicit Drugs (n = 10) | |||||||
| mean | 0.9959 | 3 | 3 | +2 | −2 | 2 | 6 |
| standard deviation | 0.0050 | ±1 | ±1 | ±15 | ±13 | ±1 | ±2 |
| median | 0.9981 | 3 | 3 | −1 | −2 | 2 | 5 |
| Pesticides (n = 37) | |||||||
| mean | 0.9943 | 7 | 4 | −1 | +1 | 2 | 5 |
| standard deviation | 0.0083 | ±6 | ±2 | ±10 | ±8 | ±1 | ±3 |
| median | 0.9982 | 5 | 4 | +1 | +2 | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egli, M.; Hartmann, A.; Rapp Wright, H.; Ng, K.T.; Piel, F.B.; Barron, L.P. Quantitative Determination and Environmental Risk Assessment of 102 Chemicals of Emerging Concern in Wastewater-Impacted Rivers Using Rapid Direct-Injection Liquid Chromatography—Tandem Mass Spectrometry. Molecules 2021, 26, 5431. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185431
Egli M, Hartmann A, Rapp Wright H, Ng KT, Piel FB, Barron LP. Quantitative Determination and Environmental Risk Assessment of 102 Chemicals of Emerging Concern in Wastewater-Impacted Rivers Using Rapid Direct-Injection Liquid Chromatography—Tandem Mass Spectrometry. Molecules. 2021; 26(18):5431. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185431
Chicago/Turabian StyleEgli, Melanie, Alicia Hartmann, Helena Rapp Wright, Keng Tiong Ng, Frédéric B. Piel, and Leon P. Barron. 2021. "Quantitative Determination and Environmental Risk Assessment of 102 Chemicals of Emerging Concern in Wastewater-Impacted Rivers Using Rapid Direct-Injection Liquid Chromatography—Tandem Mass Spectrometry" Molecules 26, no. 18: 5431. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185431