Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention
Abstract
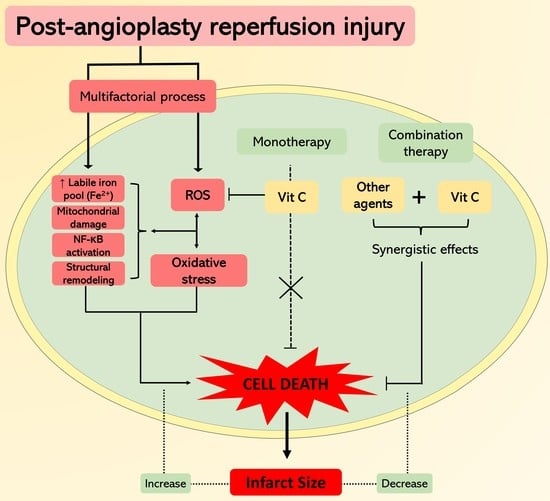
:1. Introduction
2. Ischemia-Reperfusion Induced Cardiac Injury and Oxidative Stress
2.1. Oxidative Stress Development
2.2. Antioxidant Defense System
2.3. Pathophysiology of Cardiac Ischemia/Reperfusion Injury
3. Cardioprotection by Vitamin C
3.1. Parameters Studied in Clinical Trials
3.1.1. Oxidative Stress and Inflammation Biomarkers
3.1.2. Beneficial Effects in Cardiac Function Parameters
3.1.3. Myocardial Damage Amelioration
3.1.4. Infarct Size
3.2. Antioxidant Mechanism of Vitamin C
3.3. Considerations on the Use of Vitamin C
| Model | Dose | Results | Ref. |
|---|---|---|---|
| Human Models | |||
| Human (in vivo) | 16.6 mg/min, over 1 h before PCI | Better preservation of CF No changes in HR, cTnT and MAP Better perfusion ↓ CK-MB levels ↓ Oxidative stress | [65] |
| Human (in vivo) | 500 mg, twice a day for 5 days before analysis | ↑ SOD activity ↑ Thiol levels ↓ XO activity ↓ MDA | [63] |
| Human (in vivo) | Multivitamin therapy Before reperfusion: Vit C, 1 g in IV bolus. After reperfusion (daily, for 1 month, via oral): Vit C 1 g, Vit A 50,000 Unit, Vit E 300 mg | Better preservation of cardiac function ↓ Oxidative stress ↑ Antioxidant status | [16] |
| Human (in vivo) | Initial dose of 2000 mg followed by a constant infusion at 20 mg/min before PCI | No suppression of oxidative stress | [68] |
| Human (in vivo) | 1 g/L at 24 mg/min IV infusion | Better preservation of CF cTnT was similar between control and Vit C group ↓ Oxidative stress | [66] |
| Human (in vivo) | IV infusion of 320 mM at a flow rate of 10 mL/min during the initial hour and at 3 mL/min during the following 2 h. After the primary PCI, oral doses of Vit C (500 mg/12 h) and α-tocopherol (400 IU/day) for 84 continuous days | No significant difference in infarct size between the groups Better preservation of CF No changes in CK-MB ↑ FRAP levels | [69] |
| Human (in vivo) | Initial dose of 3 g IV before PCI and 100 mg of intracoronary Vit C during PCI | ↓cTnT and CK-MB levels | [73] |
| Human (in vivo) | Initial unique oral dose of α-tocopherol (800 IU) and IV infusion of Vit C (320 mM) infused at a 10 mL/min flow rate during the first hour and at a 3 mL/min rate during the following 2 h. After the PCI, oral doses of Vit E (400 IU/day) and Vit C (500 mg/12 h) were taken by the patients for 84 continuous days. | Better preservation of CF No differences in CK-MB ↑ FRAP levels | [64] |
| Human (in vivo) | 3 g IV within 6 h before PCI | ↓ cTnT and CK-MB levels ↓ Oxidative stress | [67] |
| Animal models | |||
| Adult mongrel dogs (in vivo) | 100 mg/kg of Vit C was administered just before reperfusion | ↓ Mortality in group of supplemented dogs ↑ GSH/GSSG ratio No significant changes in activities of GPX and GR | [91] |
| Domestic pigs (in vivo) | Combined treatment of 4.4 g of Vit C (about 0.1 gm/kg) and 12 g of Vit E acetate was infused | ↓ Infarct size, but just reached the border of significance | [92] |
| Langendorff model using isolated rat hearts (ex vivo) | At the time of reperfusion one group was infused with 1 mM of AA and another group with 1 mM of AA plus 1 mM of GSHme | AA Slightly ↓ myocardial TBAR contents | [93] |
| AA plus GSHme ↑ GSH content HR and CF were recovered ↓ Incidence of VF ↓ Myocardial CK loss ↓ Myocardial TBARS content ↓ Myocardial nitrotyrosine | |||
| Young male farm pigs (in vivo) | IV infusion of 100 mg/kg AA and 60 mg/kg DFO | The therapy did not provide significant cardioprotection in the experimental group in any of the parameters measured | [94] |
| Farm-raised domestic male pigs (in vivo) | One group receive AA 100 mg/kg infusion. Other group receive AA 100 mg/kg + DFO 60 mg/kg + NAC 100 mg/kg for 20 min with a 20-mg/kg maintenance dose | The therapy did not provide significant cardioprotection in the experimental group in any of the parameters measured | [95] |
| Langendorff model using isolated male Sprague-Dawley rats hearts (ex vivo) | Hearts were post-treated with 2 μM Vit C for 30 min after global ischemia | ↓ I/R-Induced infarct area ↓ LDH activity Improved all hemodynamic variables ↑ NAD+, suggested that Vit C inhibited mPTP opening ↓ Apoptosis ↑ Oxygen consumption | [96] |
| Cell Cultures | |||
| HeLa and MCF7 cells (in vitro) | HeLa cells incubated with 1 mM DHA for 1 h and accumulated 4 mM intracellular Vit C. Conversely, cells incubated with 1 mM Vit C for 1 h accumulated 0.2 mM intracellular Vit C * | Inhibits: TNFα-induced transcriptional responses mediated by NF-κB TNF-dependent nuclear translocation of NF-κB The TNFα-induced phosphorylation and degradation of IκBR | [77] |
| Neonatal rat cardiac fibroblast (in vitro) | Cells treated with Vit C in doses of 1 μM, 10 μM 100 μM, 10,000 μM | No effect in cell viability at 1 and 10 μM ↑ Cell viability but not significantly at 100 μM ↓ Cell viability at 10,000 μM | [97] |
| Cells treated with different combinations of Vit C, DFO, NAC (Vit C/DFO, Vit C/NAC and Vit C/NAC/DFO), each in doses of 1 and 10 μM | ↑ Cell viability only Vit C/DFO in doses of 1 μM ↑ Cell viability Vit C/DFO, Vit C/NAC and Vit C/NAC/DFO in doses of 10 μM ↓ Intracellular ROS production Vit C/NAC/DFO in doses of 10 μM | ||
| HUVEC HCAEC (in vitro) | Cells were preloaded with AA by incubating with different concentrations of DHA for 30 min before subjecting to hypoxia | ↓ Apoptosis ↓ ROS levels Prevents release of Cyt C to cytosol Stabilizes mitochondrial membrane potential Inhibits procaspase-9 and procaspase-3 activation | [98] |
| Neonatal rat cardiac ventricular myocytes (in vitro) | Cells were post-conditioned with normal culture medium containing 2 μM Vit C | ↑ Cell viability ↓ LDH activity ↓Cytosolic Ca2+ overload. ↓ ROS levels Alleviated mPTP opening in cardiomyocytes Preserved ΔΨm ↑ AKT (Ser473) phosphorylation ↑ Expression of p-GSK 3β(Ser9) | [96] |
4. Cardioprotective Effects Exerted by Other Antioxidants
4.1. Vitamin E
4.2. N-Acetylcysteine
4.3. Deferoxamine
4.4. Polyphenols and Other Antioxidant Compounds
5. Towards a Potential Synergistic Cardioprotection Achieved by Combined Antioxidants
6. Discussion
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | Acute myocardial infarction |
| AREs | Antioxidant response elements |
| ATP | Adenosine triphosphate |
| BH4 | Tetrahydrobiopterin |
| C3OG | cyanidin-3-O-glucoside |
| CAT | catalase |
| cTnI | troponin I |
| cTnT | cardiac troponin T |
| CK-MB | creatinine phosphokinase MB isoenzyme |
| DFO | deferoxamine |
| DHA | dehydroascorbic acid |
| FRAP | ferric reducing ability of plasma |
| Gclc | Glutamate-Cysteine Ligase Catalytic Subunit |
| Gclm | Glutamate-Cysteine Ligase Modifier Subunit |
| GPXs | glutathione peroxidases |
| GR | glutathione reductase |
| GRXs | glutaredoxins |
| GSH | reduced glutathione |
| hs-CRP | high-sensitivity C-reactive protein |
| HO-1 | heme oxygenase-1 |
| H2O2 | hydrogen peroxide |
| I/R | ischemia/reperfusion |
| IVRT | LV isovolumic relaxation time |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDH | lactate dehydrogenase |
| LV | left ventricle |
| LVEF | LV ejection fraction |
| LVFS | LV fractional shortening |
| MDA | malondialdehyde |
| MIRI | myocardial ischemia reperfusion injury |
| MPO | myeloperoxidase |
| mPTP | mitochondrial permeability transition pore |
| NAC | N-acetylcysteine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NALP3 | NLRP3 inflammasome |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO. | nitric oxide radical |
| NOO. | nitrogen dioxide radical |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| 1O2 | oxygen singlet |
| O2._ | superoxide anion radical |
| OH | hydrogen peroxide |
| ONOO- | peroxynitrite anion |
| P3OG | pelargonidin-3-O-glucoside |
| PCI | percutaneous coronary intervention |
| PRXs | peroxiredoxins |
| ROS | reactive oxygen species |
| SODs | superoxide dismutases |
| sCD40L | soluble CD40 ligand |
| sNOX2-dp | soluble NOX2-–derived peptide |
| SVCT1 | sodium-ascorbate co-transporters 1 |
| SVCT2 | sodium-ascorbate co-transporters 2 |
| TNFα | tumor necrosis factor alpha |
| TRXs | thioredoxins |
| TxB2 | Thromboxane B2 |
| un-eNOS | uncoupled endothelial nitric oxide synthase |
| Vit C | vitamin C |
| Vit E | vitamin E |
| XO | xanthine oxidase |
References
- WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 17 May 2021).
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 17 May 2021).
- White, H.D.; Chew, D.P. Acute myocardial infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Mehta, P.; Reddivari, A.K.R.; Mungee, S. Percutaneous Coronary Intervention; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Massalha, S.; Luria, L.; Kerner, A.; Roguin, A.; Abergel, E.; Hammerman, H.; Boulos, M.; Dragu, R.; Kapeliovich, M.R.; Beyar, R.; et al. Heart failure in patients with diabetes undergoing primary percutaneous coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 455–462. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Ceccarelli, M.; Nunnari, G.; et al. Multiple effects of ascorbic acid against chronic diseases: Updated evidence from preclinical and clinical studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Bhattacharjee, S.; Ghani, M.O.A.; Walden, R.; Chen, Q.M. Vitamin c for cardiac protection during percutaneous coronary intervention: A systematic review of randomized controlled trials. Nutrients 2020, 12, 2199. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta (BBA) - Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R. Oxidative Stress and Antioxidants: Their Role in Human Disease; Rodrigo, R., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2009; ISBN 978-1-60741-554-1. [Google Scholar]
- Quinlan, C.L.; Goncalves, R.L.S.; Hey-Mogensen, M.; Yadava, N.; Bunik, V.I.; Brand, M.D. The 2-Oxoacid Dehydrogenase Complexes in Mitochondria Can Produce Superoxide/Hydrogen Peroxide at Much Higher Rates Than Complex I *. J. Biol. Chem. 2014, 289, 8312–8325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinic applications. Clin. Sci. 2013, 124, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raedschelders, K.; Ansley, D.M.; Chen, D.D.Y. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012, 133, 230–255. [Google Scholar] [CrossRef]
- Gasparetto, C.; Malinverno, A.; Culacciati, D.; Gritti, D.; Prosperini, P.G.; Specchia, G.; Ricevuti, G. Antioxidant vitamins reduce oxidative stress and ventricular remodeling in patients with acute myocardial infarction. Int. J. Immunopathol. Pharmacol. 2005, 18, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.; Strom, J.; Lee, S.; Chen, Q.M. Myocardial ischemic reperfusion induces de novo Nrf2 protein translation. Biochim. Biophys. Acta (BBA)- Mol. Basis Dis. 2014, 1842, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xiao, Z.; Yao, J.; Zhao, G.; Fa, X.; Niu, J. Participation of protein kinase C in the activation of Nrf2 signaling by ischemic preconditioning in the isolated rabbit heart. Mol. Cell. Biochem. 2013, 372, 169–179. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Balla, C.; Malagù, M.; Guardigli, G.; Morciano, G.; Bertini, M.; Biscaglia, S.; Campo, G. Reperfusion damage—A story of success, failure, and hope—. Circ. J. 2017, 81, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosio, G.; Weisfeldt, M.L.; Jacobus, W.E.; Flaherty, J.T.; Hopkins, J. Evidence for a reversible oxygen radical-mediated component of reperfusion injury: Reduction by recombinant human superoxide dismutase administered at the time of reflow EXPERIMENTAL and clinical studies have indicated From the Department of Medicine, Division of Cardiology, The that timely reperfusion of ischemic myocardium can. Lab. Investig. Myocard. Reperfus. Circ. 1987, 75, 282–291. [Google Scholar]
- Neely, J.R.; Grotyohann, L.W. Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ. Res. 1984, 55, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orchard, C.; Kentish, J. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 1990, 258, C967–C981. [Google Scholar] [CrossRef]
- Avkiran, M.; Marber, M. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: Progress, problems and prospects. J. Am. Coll. Cardiol. 2002, 39, 747–753. [Google Scholar] [CrossRef]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve 2006, 33, 715–731. [Google Scholar] [CrossRef]
- Bernardi, P.; Vassanelli, S.; Veronese, P.; Colonna, R.; Szabo, I.; Zoratti, M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem. 1992, 267, 2934–2939. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef] [Green Version]
- Braunersreuther, V.; Jaquet, V. Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Curr. Pharm. Biotechnol. 2012, 13, 97–114. [Google Scholar] [CrossRef] [PubMed]
- González-Montero, J.; Brito, R.; Gajardo, A.I.; Rodrigo, R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenshtein, E.; Vaisman, B.; Goldberg-Langerman, C.; Kitrossky, N.; Konijn, A.M.; Chevion, M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol. Cell. Biochem. 2002, 234, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wu, S.; Wong, T.M.; Chung, S.K.; Chung, S.S.M. Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free Radic. Biol. Med. 2008, 45, 602–610. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: Relation to microvascular obstruction and prognostic significance. Circ. Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef] [Green Version]
- Vernis, L.; El Banna, N.; Baïlle, D.; Hatem, E.; Heneman, A.; Huang, M.E. Fe-S Clusters Emerging as Targets of Therapeutic Drugs. Oxid. Med. Cell. Longev. 2017, 2017, 3647657. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Moya, J.; Rojas-Solé, C.; Muñoz-Salamanca, D.; Panieri, E.; Saso, L.; Rodrigo, R. Targeting ferroptosis against ischemia/reperfusion cardiac injury. Antioxidants 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J.; Halestrap, A.P. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995, 307, 93. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jin, Y.; Lemasters, J. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2024–H2034. [Google Scholar] [CrossRef] [PubMed]
- Seidlmayer, L.; Juettner, W.; Kettlewell, S.; Pavlov, E.; Blatter, L.; Dedkova, E. Distinct mPTP activation mechanisms in ischaemia-reperfusion: Contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 2015, 106, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Kitakaze, M.; Takashima, S.; Funaya, H.; Minamino, T.; Node, K.; Shinozaki, Y.; Mori, H.; Hori, M. Temporary acidosis during reperfusion limits myocardial infarct size in dogs. Am. J. Physiol. 1997, 272, H2071–H2078. [Google Scholar] [CrossRef]
- Baines, C.P.; Molkentin, J.D. Adenine nucleotide translocase-1 induces cardiomyocyte death through upregulation of the pro-apoptotic protein Bax. J. Mol. Cell. Cardiol. 2009, 46, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, P. The mitochondrial permeability transition pore: A mystery solved? Front. Physiol. 2013, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Maddock, H.; Baxter, G.; Yellon, D.M. Inhibiting mitochondrial permeability transition pore opening: A new paradigm for myocardial preconditioning? Cardiovasc. Res. 2002, 55, 534–543. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Duchen, M.; Yellon, D.M. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc. Res. 2003, 60, 617–625. [Google Scholar] [CrossRef]
- Argaud, L.; Gateau-Roesch, O.; Muntean, D.; Chalabreysse, L.; Loufouat, J.; Robert, D.; Ovize, M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J. Mol. Cell. Cardiol. 2005, 38, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Skyschally, A.; Schulz, R.; Heusch, G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc. Drugs Ther. 2010, 24, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Parra, P.; Rodrigo, R. Novel Antioxidant Therapy against Myocardial Ischemia–Reperfusion Injury during Percutaneous Coronary Angioplasty. In Free Radicals and Diseases; Ahmad, R., Ed.; InTech: London, UK, 2016. [Google Scholar]
- Fan, H.; Sun, B.; Gu, Q.; Lafond-Walker, A.; Cao, S.; Becker, L. Oxygen radicals trigger activation of NF-kappaB and AP-1 and upregulation of ICAM-1 in reperfused canine heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1778–H1786. [Google Scholar] [CrossRef] [Green Version]
- Bowie, A.; O’Neill, L. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Freeman, G. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997, 401, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Feng, G.; Gauthier, J.M.; Lokshina, I.; Higashikubo, R.; Evans, S.; Liu, X.; Hassan, A.; Tanaka, S.; Cicka, M.; et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Investig. 2019, 129, 2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, C.; Zúñiga, F.; Salas-Burgos, A.; Mardones, L.; Ormazabal, V.; Vera, J. Vitamin C transporters. J. Physiol. Biochem. 2008, 64, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Grover-McKay, M.; Walsh, S.; Thompson, S. Glucose transporter 3 (GLUT3) protein is present in human myocardium. Biochim. Biophys. Acta 1999, 1416, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-van Straaten, H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Annu. Update Intensive Care Emerg. Med. 2018, 22, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A.; Colucci, R.; Fornai, M.; Polini, A.; Daghini, E.; Duranti, E.; Ghisu, N.; Versari, D.; Dardano, A.; Blandizzi, C.; et al. Inducible nitric oxide synthase is involved in endothelial dysfunction of mesenteric small arteries from hypothyroid rats. Endocrinology 2009, 150, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Salvetti, A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998, 97, 2222–2229. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Delles, C.; Schmidt, B.; Oehmer, S.; Schwarz, T.; Schmieder, R.; John, S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005, 18, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Bhakuni, P.; Chandra, M.; Misra, M. Effect of ascorbic acid supplementation on certain oxidative stress parameters in the post reperfusion patients of myocardial infarction. Mol. Cell. Biochem. 2006, 290, 153–158. [Google Scholar] [CrossRef]
- Valls, N.; Gormaz, J.G.; Aguayo, R.; González, J.; Brito, R.; Hasson, D.; Libuy, M.; Ramos, C.; Carrasco, R.; Prieto, J.C.; et al. Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep. 2016, 21, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Basili, S.; Tanzilli, G.; Mangieri, E.; Raparelli, V.; Di Santo, S.; Pignatelli, P.; Violi, F. Intravenous Ascorbic Acid Infusion Improves Myocardial Perfusion Grade During Elective Percutaneous Coronary Intervention: Relationship With Oxidative Stress Markers. JACC Cardiovasc. Interv. 2010, 3, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Tanzilli, G.; Carnevale, R.; Di Santo, S.; Loffredo, L.; Celestini, A.; Proietti, M.; Tovaglia, P.; Mangieri, E.; Basili, S.; et al. Ascorbic Acid Infusion Blunts CD40L Upregulation in Patients Undergoing Coronary Stent. Cardiovasc. Ther. 2011, 29, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Hu, W.K.; Liu, Y.Y.; Shi, D.M.; Cheng, W.J.; Guo, Y.H.; Yang, Q.; Zhao, Y.X.; Zhou, Y.J. The Effect of Intravenous Vitamin C Infusion on Periprocedural Myocardial Injury for Patients Undergoing Elective Percutaneous Coronary Intervention. Can. J. Cardiol. 2014, 30, 96–101. [Google Scholar] [CrossRef]
- Guan, W.; Osanai, T.; Kamada, T.; Ishizaka, H.; Hanada, H.; Okumura, K. Time Course of Free Radical Production After Primary Coronary Angioplasty for Acute Myocardial Infarction and the Effect of Vitamin C. Jpn. Circ. J. 1999, 63, 924–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, C.; Brito, R.; González-Montero, J.; Valls, N.; Gormaz, J.G.; Prieto, J.C.; Aguayo, R.; Puentes, Á.; Noriega, V.; Pereira, G.; et al. Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch. Med. Sci. 2017, 13, 558. [Google Scholar] [CrossRef]
- Akolkar, G.; da Silva Dias, D.; Ayyappan, P.; Bagchi, A.K.; Jassal, D.; Salemi, V.; Irigoyen, M.; De Angelis, K.; Singal, P. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Solaro, R.; Arteaga, G. Heart failure, ischemia/reperfusion injury and cardiac troponin. Adv. Exp. Med. Biol. 2007, 592, 191–200. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.; Jaffe, A.; Chaitman, B.; Bax, J.; Morrow, D.; White, H. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Shafaei-Bajestani, N.; Talasaz, A.; Salarifar, M.; Pourhosseini, H.; Sadri, F.; Jalali, A. Potential Role of Vitamin C Intracoronary Administration in Preventing Cardiac Injury After Primary Percutaneous Coronary Intervention in Patients with ST-Elevation Myocardial Infarction. J. Res. Pharm. Pract. 2019, 8, 75–82. [Google Scholar] [CrossRef]
- Jackson, T.; Xu, A.; Vita, J.; Keaney, J. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998, 83, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Molecular basis of cardioprotective effect of antioxidant vitamins in myocardial infarction. Biomed Res. Int. 2013, 2013, 437613. [Google Scholar] [CrossRef] [Green Version]
- Ulker, S.; McKeown, P.; Bayraktutan, U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension 2003, 41, 534–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárcamo, J.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef] [PubMed]
- Newaz, M.A.; Yousefipour, Z.; Nawal, N.N.A. Modulation of nitric oxide synthase activity in brain, liver, and blood vessels of spontaneously hypertensive rats by ascorbic acid: Protection from free radical injury. Clin. Exp. Hypertens. 2005, 27, 497–508. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.-F. Recent Advances in Understanding Endothelial Dysfunction in Atherosclerosis. Clin. Med. Res. 2006, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, J.; Slater, T.; Willson, R. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979, 278, 737–738. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N.; Tsuchihashi, H.; Gotoh, N. Interaction among vitamin C, vitamin E, and beta-carotene. Am. J. Clin. Nutr. 1995, 62, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Qu, Z.; Mendiratta, S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998, 349, 281–289. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Graumlich, J.; Ludden, T.; Conry-Cantilena, C.; Cantilena, L.; Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 1997, 14, 1133–1139. [Google Scholar] [CrossRef]
- Padayatty, S.; Sun, A.; Chen, Q.; Espey, M.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [Green Version]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.; Qu, Z.; Whitesell, R.; Cobb, C. Ascorbate recycling in human erythrocytes: Role of GSH in reducing dehydroascorbate. Free Radic. Biol. Med. 1996, 20, 543–551. [Google Scholar] [CrossRef]
- Mendiratta, S.; Qu, Z.; May, J. Enzyme-dependent ascorbate recycling in human erythrocytes: Role of thioredoxin reductase. Free Radic. Biol. Med. 1998, 25, 221–228. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.C.; He, F.; Cheng, C.; Xu, B.L.; Sheng, J.L. Uric acid aggravates myocardial ischemia–reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 2021, 133, 110990. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, Y.; Sugiyama, S.; Yokota, M.; Saito, H.; Ozawa, T. The effects of a high dose of ascorbate on ischemia-reperfusion-induced mitochondrial dysfunction in canine hearts. Heart Vessels 1992, 7, 18–23. [Google Scholar] [CrossRef]
- Klein, H.H.; Pich, S.; Lindert, S.; Nebendahl, K.; Niedmann, P.; Kreuzer, H. Combined treatment with vitamins E and C in experimental myocardial infarction in pigs. Am. Heart J. 1989, 118, 667–673. [Google Scholar] [CrossRef]
- Gao, F.; Yao, C.L.; Gao, E.; Mo, Q.Z.; Yan, W.L.; McLaughlin, R.; Lopez, B.L.; Christopher, T.A.; Ma, X.L. Enhancement of glutathione cardioprotection by ascorbic acid in myocardial reperfusion injury. J. Pharmacol. Exp. Ther. 2002, 301, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Chatziathanasiou, G.N.; Nikas, D.N.; Katsouras, C.S.; Kazakos, N.D.; Bouba, V.; Vougiouklakis, T.; Naka, K.K.; Michalis, L.K. Combined intravenous treatment with ascorbic acid and desferrioxamine to reduce myocardial reperfusion injury in an experimental model resembling the clinical setting of primary PCI. Hell. J. Cardiol. 2012, 53, 195–204. [Google Scholar]
- Nikas, D.N.; Chatziathanasiou, G.; Kotsia, A.; Papamichael, N.; Thomas, C.; Papafaklis, M.; Naka, K.K.; Kazakos, N.; Milionis, H.J.; Vakalis, K.; et al. Effect of intravenous administration of antioxidants alone and in combination on myocardial reperfusion injury in an experimental pig model. Curr. Ther. Res. - Clin. Exp. 2008, 69, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Li, W.W.; Du, H.; Zhao, Z.F.; Liu, F.; Lu, J.C.; Yang, X.C.; Cui, W. Role of Vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial katp channel. Chem. Pharm. Bull. 2016, 64, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Parra-Flores, P.; Riquelme, J.A.; Valenzuela-Bustamante, P.; Leiva-Navarrete, S.; Vivar, R.; Cayupi-Vivanco, J.; Castro, E.; Espinoza-Pérez, C.; Ruz-Cortés, F.; Pedrozo, Z.; et al. The association of ascorbic acid, deferoxamine and N-acetylcysteine improves cardiac fibroblast viability and cellular function associated with tissue repair damaged by simulated ischemia/reperfusion. Antioxidants 2019, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Dhar-Mascareño, M.; Cárcamo, J.M.; Golde, D.W. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005, 38, 1311–1322. [Google Scholar] [CrossRef]
- Watanabe, M.; Okada, T. Langendorff Perfusion Method as an Ex Vivo Model to Evaluate Heart Function in Rats. Methods Mol. Biol. 2018, 1816, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Halapas, A.; Papalois, A.; Stauropoulou, A.; Philippou, A.; Pissimissis, N.; Chatzigeorgiou, A.; Kamper, E.; Koutsilieris, M. In vivo models for heart failure research. In Vivo 2008, 22, 767–780. [Google Scholar]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yin, C.; Yang, Y.; Fan, Z.; Shang, J.; Tan, W. Inhibition of rapid delayed rectifier potassium current (I Kr) by ischemia/reperfusion and its recovery by vitamin E in ventricular myocytes. J. Electrocardiol. 2017, 50, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Hildebrand, L.; Schirdewahn, C.; Eitel, I.; Adams, V.; Fuernau, G.; Erbs, S.; Linke, A.; Diederich, K.W.; Nowak, M.; et al. Impact of High-Dose N-Acetylcysteine Versus Placebo on Contrast-Induced Nephropathy and Myocardial Reperfusion Injury in Unselected Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: The LIPSIA-. J. Am. Coll. Cardiol. 2010, 55, 2201–2209. [Google Scholar] [CrossRef] [Green Version]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-Acetylcysteine in Combination With Nitroglycerin and Streptokinase for the Treatment of Evolving Acute Myocardial Infarction. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Nozari, Y.; Eshraghi, A.; Talasaz, A.H.; Bahremand, M.; Salamzadeh, J.; Salarifar, M.; Pourhosseini, H.; Jalali, A.; Mortazavi, S.H. Protection from Reperfusion Injury with Intracoronary N -Acetylcysteine in Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention in a Cardiac Tertiary Center. Am. J. Cardiovasc. Drugs 2018, 18, 213–221. [Google Scholar] [CrossRef]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Kapan, S.; Turker, Y.; Varol, E.; Ozguner, F.; Dogan, A.; Ibrisim, E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: A prospective, randomized, placebo-controlled pilot study. Eur. Heart J. 2008, 29, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskevaidis, I.A.; Iliodromitis, E.K.; Vlahakos, D.; Tsiapras, D.P.; Nikolaidis, A.; Marathias, A.; Michalis, A.; Kremastinos, D.T. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: Immediate and long-term significance. Eur. Heart J. 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Taylor, A.J.; Ellims, A.H.; Lefkovits, L.; Wong, C.; Kingwell, B.A.; Natoli, A.; Croft, K.D.; Mori, T.; Kaye, D.M.; et al. Effect of iron chelation on myocardial infarct size and oxidative stress in ST-elevation-myocardial infarction. Circ. Cardiovasc. Interv. 2012, 5, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakanashi, Y.; Oyama, K.; Matsui, H.; Oyama, T.; Oyama, T.; Nishimura, Y.; Sakai, H.; Oyama, Y. Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular Ca2+: A model experiment. Life Sci. 2008, 83, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Carnická, S.; Pancza, D.; Ondrejcáková, M.; Breier, A.; Ravingerová, T. Acute treatment with polyphenol quercetin improves postischemic recovery of isolated perfused rat hearts after global ischemia. Can. J. Physiol. Pharmacol. 2010, 88, 465–471. [Google Scholar] [CrossRef]
- Shoskes, D.; Zeitlin, S.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Li, J.; Xie, C.; Zhuang, J.; Li, H.; Yao, Y.; Shao, C.; Wang, H. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: Role of the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2015, 11, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar]
- Dong, W.; Yang, R.; Yang, J.; Yang, J.; Ding, J.; Wu, H.; Zhang, J. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8731–8741. [Google Scholar]
- Škemiene, K.; Jablonskiene, G.; Liobikas, J.; Borutaite, V. Protecting the heart against ischemia/reperfusion-induced necrosis and apoptosis: The effect of anthocyanins. Medicina 2013, 49, 84–88. [Google Scholar] [CrossRef]
- Nakamura, T.; Goto, M.; Matsumoto, A.; Tanaka, I. Inhibition of NF-kappa B transcriptional activity by alpha-tocopheryl succinate. Biofactors 1998, 7, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lassnigg, A.; Punz, A.; Barker, R.; Keznickl, P.; Manhart, N.; Roth, E.; Hiesmayr, M. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: A double-blinded, randomized, controlled study. Br. J. Anaesth. 2003, 90, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Evidence for beneficial effects of vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar] [CrossRef]
- Miller Iii, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality Background: Experimental models and observational studies. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Mittal, D.K.; Shrivastava, S.; Shukla, S. Protective role of thiol chelators against dimethylmercury induced toxicity in male rats. Bull. Environ. Contam. Toxicol. 2010, 84, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, W.; Shen, T.; Dou, L.; Man, Y.; Wang, S.; Xiao, C.; Li, J. The antioxidant N-acetylcysteine promotes atherosclerotic plaque stabilization through suppression of rage, MMPs and NF-κB in ApoE-deficient Mice. J. Atheroscler. Thromb. 2011, 18, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM trial [N-acetylcysteine in acute myocardial infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef]
- Rhodes, K.; Braakhuis, A. Performance and Side Effects of Supplementation with N-Acetylcysteine: A Systematic Review and Meta-Analysis. Sport. Med. 2017, 47, 1619–1636. [Google Scholar] [CrossRef]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [Green Version]
- Riegman, M.; Bradbury, M.S.; Overholtzer, M. Population Dynamics in Cell Death: Mechanisms of Propagation. Trends in Cancer 2019, 5, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.J.; Luo, X.J.; Tu, H.; Chen, H.; Xiong, X.M.; Li, N.S.; Peng, J. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 401–410. [Google Scholar] [CrossRef]
- Howland, M.A. Risks of parenteral deferoxamine for acute iron poisoning. Clin. Toxicol. 1996, 34, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Donfrancesco, A.; Deb, G.; Dominici, C.; Pileggi, D.; Castello, M.A.; Helson, L. Effects of a Single Course of Deferoxamine in Neuroblastoma Patients. Cancer Res. 1990, 50, 4929–4930. [Google Scholar] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; DeTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Mendonça, R.; Carvalho, N.; Martin-Moreno, J.; Pimenta, A.; Lopes, A.; Gea, A.; Martinez-Gonzalez, M.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Van Jaarsveld, H.; Kuyl, J.; Schulenburg, D.; Wiid, N. Effect of flavonoids on the outcome of myocardial mitochondrial ischemia/reperfusion injury. Res. Commun. Mol. Pathol. Pharmacol. 1996, 95, 65–75. [Google Scholar]
- Brosková, Z.; Drábiková, K.; Sotníková, R.; Fialová, S.; Knezl, V. Effect of Plant Polyphenols on Ischemia-Reperfusion Injury of the Isolated rat Heart and Vessels. Phyther. Res. 2013, 27, 1018–1022. [Google Scholar] [CrossRef]
- Cebova, M.; Pechanova, O. Protective Effects of Polyphenols against Ischemia/Reperfusion Injury. Molecules 2020, 25, 3469. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, B.; Reznikov, L.; Harken, A.; Dinarello, C. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc. Natl. Acad. Sci. USA 2001, 98, 2871–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, L.; Chen, J.; Lee, R.; Liang, H.; Su, M. Beneficial effects of astringinin, a resveratrol analogue, on the ischemia and reperfusion damage in rat heart. Free Radic. Biol. Med. 2001, 30, 877–883. [Google Scholar] [CrossRef]
- Hung, L.; Chen, J.; Huang, S.; Lee, R.; Su, M. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000, 47, 549–555. [Google Scholar] [CrossRef]
- De Souza, R.F.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2013, 9, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausenloy, D.J.; Garcia-Dorado, D.; Bøtker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef] [Green Version]
- Verhoef, T.; Morris, S.; Mathur, A.; Singer, M. Potential economic consequences of a cardioprotective agent for patients with myocardial infarction: Modelling study. BMJ Open 2015, 5, e008164. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Hasson, D.; Prieto, J.C.; Dussaillant, G.; Ramos, C.; León, L.; Gárate, J.; Valls, N.; Gormaz, J.G. The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC Trial): Study protocol for a pilot randomized double-blind controlled trial. Trials 2014, 15, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; González-Montero, J.; Sotomayor, C.G. Novel combined antioxidant strategy against hypertension, acute myocardial infarction and postoperative atrial fibrillation. Biomedicines 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]







| Antioxidant | Model | Dose | Results | Ref. | |
|---|---|---|---|---|---|
| Vit E α-tocopherol (C29H50O2) | C57BL/6 mice (in vivo) | 2.5 mg/kg BW in 0.8% DMSO 2 h prior to surgery, immediately after PCI, and twice per day for three consecutive days | ↓ Infarct size ↓ ROS and lipid oxidation ↓ MPO activity ↓ Neutrophil infiltration Prevented pathological changes | [101] | |
| Langendorff model using male Hartley Guinea pigs hearts (ex vivo) | 100 μM | QT segment recovered 10% | [102] | ||
| NAC (C5H9NO3S) NAC (C5H9NO3S) | Human (in vivo) | IV bolus of 1200 mg before PCI and 1200 mg IV twice daily for the 48 h after PCI (total dose 6000 mg) | ↓ Oxidative stress It does not provide an additional clinical benefit to placebo with respect to patients undergoing PCI. No adverse effects. | [103] | |
| Human (in vivo) | Patients with AMI received 15 g infused over 24 h + IV NTG and streptokinase | ↓ Oxidative stress. NAC and GSH concentration were correlated. ↓ MDA concentrations over the first 8 h of treatment. Better preservation of LV function. No adverse effects. | [104] | ||
| Human (in vivo) | NAC 100 mg/kg bolus followed by intracoronary NAC 480 mg during PCI then IV NAC 10 mg/kg for 12 h | ↓ Peak hs-TnT level after PCI. Difference in peak CK-MB was not statistically significant. No adverse effects | [105] | ||
| Human (in vivo) | Infusion of 50 mg/kg, followed by IV infusion for 48 h after the operation at a dose of 50 mg/kg/day | ↓ Rate of atrial fibrillation in the NAC group. | [106] | ||
| DFO (C25H48N6O8) | Human (in vivo) | 4 g were infused for 8 h | Prevented ROS production Improved LVEF No major cardiac event was reported with long term administration | [107] | |
| Human (in vivo) | 500 mg 5 to 10 min before PCI, followed by 50 mg/kg over 12 h | ↓ Serum iron and plasma F2-isoprostane levels during the first hours. No changes in the infarct size. | [108] | ||
| Polyphenols (Flavonols) | Quercetin (C15H10O7) | Cells suspension of rats (Wistar strain) Thymocytes (in vitro) | 2 mL cell suspension in a 10 mL test tube | Protective effect on the cells suffering oxidative stress and cells suffering from intracellular Ca2+ overload. ↓ Cell death | [109] |
| Langendorff model using male Wistar rat hearts (ex vivo) | 15 µM | Improvement in the functional parameters of the heart (LVDP and contractility) ↓ End-diastolic pressure. | [110] | ||
| Human (in vivo) | 500 mg twice daily for 1 month | ↓ Inflammation | [111] | ||
| Polyphenols (Stilbenes) | Resveratrol (C14H12O3) | Male rats (Sprague-Dawley) (in vivo) | 100 µM | ↓ Infarct size, ↓ Myocardial apoptosis ↓ NF-κB expression ↓ Neutrophil infiltration ↓ TNF-α levels ↓ Cardiac dysfunction ↓ Activity of serum CK-MB and LDH level ↓ MDA levels ↑ Antioxidant enzymes activities ↑ Nrf2 and HO-1 | [112,113] |
| Male rats (Sprague-Dawley) (in vivo) | 2.5, 5, and 10 mg/kg | ↓ Necrotic area ↓ TnT and CK-MB release ↓ IL-1β and IL-18 release ↓ Myocardial NALP3 expression Inhibits I/R-mediated myocardial Caspase1 expression. | [114] | ||
| Polyphenols (Anthocyanins) | C3OG (C21H21O11+, Cl−) | Langendorff model (ex vivo) using hearts from male rats (Wistar) | 20 μM | ↓ Cardiomyocyte death ↓ LDH levels Protection against apoptosis induced by I/R Cytochrome c-reducing activity Stimulation of mitochondrial respiration after ischemia | [115] |
| 40 μM | No significant changes compared to 20 μM of C3OG | ||||
| P3OG (C21H21O10+) | Langendorff model (ex vivo) using hearts from male rats (Wistar) | 20 μM | Cardiomyocyte death was not statistically different from the I/R control group ↑ LDH activity than in the control group and similar to the I/R group | ||
| 40 μM | No significant changes compared to 20 μM of P3GO | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, Á.; Gajardo, A.; Panieri, E.; Rojas-Solé, C.; Lillo-Moya, J.; Saso, L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules 2021, 26, 5702. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185702
Rodrigo R, Prieto JC, Aguayo R, Ramos C, Puentes Á, Gajardo A, Panieri E, Rojas-Solé C, Lillo-Moya J, Saso L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules. 2021; 26(18):5702. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185702
Chicago/Turabian StyleRodrigo, Ramón, Juan Carlos Prieto, Rubén Aguayo, Cristóbal Ramos, Ángel Puentes, Abraham Gajardo, Emiliano Panieri, Catalina Rojas-Solé, José Lillo-Moya, and Luciano Saso. 2021. "Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention" Molecules 26, no. 18: 5702. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185702