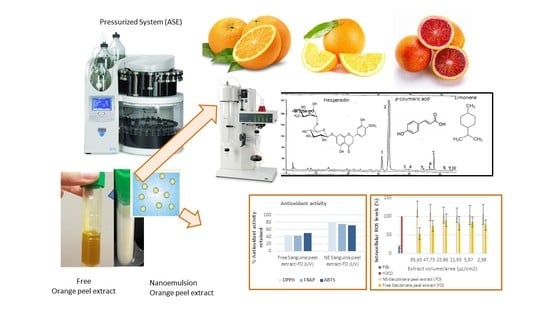
Pressurized Extraction as an Opportunity to Recover Antioxidants from Orange Peels: Heat treatment and Nanoemulsion Design for Modulating Oxidative Stress
Abstract
:1. Introduction
2. Material and Method
2.1. Material: Orange Peel and Cell Line
2.2. Heat Treatment: Oven-Drying and Freeze-Drying of Orange Peels
2.3. Isolation of Volatile Compounds by ASE and GC-MS Analysis
2.4. Isolation of Individual Flavonoids by ASE and HPLC-DAD-ESI-MS Analysis
2.5. Determination of Phenolic Acids
2.6. Determination of Total Phenolic Content
2.7. Determination of Total Flavonoids Content
2.8. Antioxidant Activity of Orange Peels
2.8.1. DPPH Radical Scavenging Assay
2.8.2. ABTS•+ Radical Scavenging Assay
2.8.3. FRAP Assay
2.9. Formulation of Nanoemulsion Systems from Sanguina Peel Extracts
2.10. In Vitro Studies in Caco-2: Nanoemulsion Viability and Intracellular ROS Levels Measurements
2.10.1. Cell Culture
2.10.2. Nanoemulsions Effect on Cell Viability
2.10.3. Intracellular ROS Levels Measurements
2.11. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds from Orange Peels
3.2. Total Phenolic Content
3.3. Phenolic Acids
3.4. Total Flavonoids Content
3.5. Individual Flavonoids of Orange Peel Extracts
3.6. Effect of Drying Treatments on the Antioxidant Activity of Orange Peels
3.7. Nanoemulsion Systems from Orange Peels
3.8. In Vitro Studies in Caco-2: Nanoemulsions’ Effects on Cell Viability
3.9. In Vitro Studies in Caco-2: Inhibition of Intracellular ROS Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): “In vivo” studies. J. Funct. Foods 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Azman, N.F.I.N.; Azlan, A.; Khoo, H.E.; Razman, M.R. Antioxidant Properties of Fresh and Frozen Peels of Citrus Species. Curr. Res. Nutr. Food Sci. J. 2019, 7, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Abudayeh, Z.H.; Al Khalifa, I.I.; Mohammed, S.M.; Ahmad, A.A. Phytochemical Content and Antioxidant Activities of Pomelo Peel Extract. Pharmacogn. Res. 2019, 11, 244–247. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview onCitrus aurantiumL.: Its Functions as Food Ingredient and Therapeutic Agent. Oxidative Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, V.; Aggarwal, A.; Kumar, A. Protective effect of naringin against ischemic reperfusion cerebral injury: Possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur. J. Pharmacol. 2009, 616, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, Q.; Wu, H.; Liu, C.; Hu, J.; Zhang, D.; Xu, C. Effects of naringin on learning and memory dysfunction induced by gp120 in rats. Brain Res. Bull. 2016, 124, 164–171. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Zhu, X.; Wu, W.; Wang, Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Nwoke, E.E.; Aderibigbe, A.O.; Omogbiya, I.A.; Ajayi, A.M.; Olonode, E.T.; Umukoro, S.; Iwalewa, E.O. Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed. Pharmacother. 2018, 109, 536–546. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020, 134, 109228. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Cho Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: Comparison with hot water and organic solvent extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Liang, Z.; Li, S.; Cai, J.; Zhu, Z.; Liu, G. HPLC-DAD-ESI-MS2 analysis of phytochemicals from Sichuan red orange peel using ultrasound-assisted extraction. Food Biosci. 2018, 25, 15–20. [Google Scholar] [CrossRef]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Meklati, B.Y.; Chemat, F. A new process for extraction of essential oil from Citrus peels: Microwave hydrodiffusion and gravity. J. Food Eng. 2009, 90, 409–413. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2016, 34, 29–46. [Google Scholar] [CrossRef]
- M’Hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2016, 33, 587–619. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Jambrak, A.R.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Trabelsi, D.; Aydi, A.; Zibetti, A.W.; Della Porta, G.; Scognamiglio, M.; Cricchio, V.; Langa, E.; Abderrabba, M.; Mainar, A.M. Supercritical extraction from Citrus aurantium amara peels using CO2 with ethanol as co-solvent. J. Supercrit. Fluids 2016, 117, 33–39. [Google Scholar] [CrossRef]
- LoPresto, C.G.; Meluso, A.; Di Sanzo, G.; Chakraborty, S.; Calabrò, V. Process-intensified waste valorization and environmentally friendly d-limonene extraction. Euro-Mediterr. J. Environ. Integr. 2019, 4, 31. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Lim, D.J.; Chun, B.S. Antioxidant and antimicrobial activity of oils obtained from a mixture of citrus by-products using a modified supercritical carbon dioxide. J. Ind. Eng. Chem. 2018, 57, 339–348. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.; Torres-Mayanga, P.C.; Maróstica, M.R.; Rostagno, M.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Omar, J.; Alonso, I.; Garaikoetxea, A.; Etxebarria, N. Optimization of Focused Ultrasound Extraction (FUSE) and Supercritical Fluid Extraction (SFE) of Citrus Peel Volatile Oils and Antioxidants. Food Anal. Methods 2012, 6, 1244–1252. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheigh, C.-I.; Chung, E.-Y.; Chung, M.-S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Kim, J.-W.; Nagaoka, T.; Ishida, Y.; Hasegawa, T.; Kitagawa, K.; Lee, S.-C. Subcritical Water Extraction of Nutraceutical Compounds from Citrus Pomaces. Sep. Sci. Technol. 2009, 44, 2598–2608. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Barrales, F.M.; Silveira, P.; Barbosa, P.D.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez Mdel, M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxidative Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsirigotis-Maniecka, M.; Lamch, L.; Chojnacka, I.; Gancarz, R.; Wilk, K.A. Microencapsulation of hesperidin in polyelectrolyte complex microbeads: Physico-chemical evaluation and release behavior. J. Food Eng. 2017, 214, 104–116. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Bioavailability of nanotechnology-based bioactives and nutraceuticals. Adv. Food Nutr. Res. 2019, 88, 235–273. [Google Scholar]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and delivery of bioactive citrus pomace polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Rodríguez-Robledo, V.; Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V.; González, J.; Villaseca, N.; Marcos, P.; Arroyo-Jiménez, M.M. Pressurized liquid extraction to obtain chia seeds oils extracts enriched in tocochromanols. Nanoemulsions approaches to preserve the antioxidant potential. J. Food Sci. Technol. 2021, 58, 4034–4044. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2011, 3, 202–220. [Google Scholar] [CrossRef]
- Oliver, M.P.; Santander-Ortega, M.; Castro-Vázquez, L.; Robledo, V.R.; González-Fuentes, J.; Marcos, P.; Lozano, M.; Arroyo-Jiménez, M. The role of the intestinal-protein corona on the mucodiffusion behaviour of new nanoemulsions stabilised by ascorbyl derivatives. Colloids Surf. B Biointerfaces 2019, 186, 110740. [Google Scholar] [CrossRef] [PubMed]
- Santalices, I.; Torres, D.; Lozano, M.V.; Arroyo-Jiménez, M.M.; Alonso, M.J.; Santander-Ortega, M.J. Influence of the surface properties of nanocapsules on their interaction with intestinal barriers. Eur. J. Pharm. Biopharm. 2018, 133, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Oliver, M.; Cano, E.L.; Arroyo-Jimenez, M.M.; Gámez, M.; Lozano-López, M.V.; Santander-Ortega, M.J. Taking Particle Tracking into Practice by Novel Software and Screening Approach: Case-Study of Oral Lipid Nanocarriers. Pharmaceutics 2021, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yuanwen, W.; Xu, X. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2016, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Chen, J.; Tian, G.; McClements, D.J.; Xiao, H.; Zheng, J. Encapsulation of Polymethoxyflavones in Citrus Oil Emulsion-Based Delivery Systems. J. Agric. Food Chem. 2017, 65, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.; Pérez-Coello, M. Aromatic potential of Castanea sativa Mill. compared to Quercus species to be used in cooperage. Food Chem. 2011, 130, 875–881. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.E.; Ricardo-Da-Silva, J.M.; Pérez-Coello, M.S.; Laureano, O. Evaluation of Portuguese and Spanish Quercus pyrenaica and Castanea sativa species used in cooperage as natural source of phenolic compounds. Eur. Food Res. Technol. 2013, 237, 367–375. [Google Scholar] [CrossRef]
- Singleton, V.L. Citation classic—Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Curr. Contents/Agric. Biol. Environ. Sci. 1985, 48, 18. [Google Scholar]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafezi, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Oikeh, E.I.; Ayevbuomwan, M.; Irabor, F.; Oikeh, A.O.; Oviasogie, F.E.; Omoregie, E.S. Evaluation of the Phenolic Content, Antioxidant and Antimicrobial Activities of Oil and Non-Oil Extracts of Citrus sinensis (L.) Osbeck Seeds. Prev. Nutr. Food Sci. 2020, 25, 280–285. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.; Hermosín-Gutiérrez, I.; Gordon, M.H.; Pérez-Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590. [Google Scholar] [CrossRef]
- Lozano, M.V.; Lollo, G.; Alonso-Nocelo, M.; Brea, J.; Vidal, A.; Torres, D.; Alonso, M.J. Polyarginine nanocapsules: A new platform for intracellular drug delivery. J. Nanoparticle Res. 2013, 15, 1515. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci. Nutr. 2020, 8, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Samadi, L.; Larijani, K.; Badi, H.N.; Mehrafarin, A. Qualitative and quantitative variations of the essential oils of Dracocephalum kotschyi Boiss. as affected by different drying methods. J. Food Process. Preserv. 2018, 42, e13816. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; de la Fuente, E.; Pérez-Coello, M.S. Influence of storage conditions on chemical composition and sensory properties of citrus honey. J. Agric. Food Chem. 2008, 56, 1999–2006. [Google Scholar] [CrossRef]
- de Torres, C.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wolken, W.A.M.; Have, R.T.; Van Der Werf, M.J. Amino acid-catalyzed conversion of citral: Cis-trans isomerization and its conversion into 6-methyl-5-hepten-2-one and acetaldehyde. J. Agric. Food Chem. 2000, 48, 5401–5405. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wen, H.; Chen, J.; Peng, Z.; Shi, M.; Chen, M.; Yuan, Z.; Liu, Y.; Zhang, H.; Xu, J. Volatile Compounds in Fruit Peels as Novel Biomarkers for the Identification of Four Citrus Species. Molecules 2019, 24, 4550. [Google Scholar] [CrossRef] [Green Version]
- Jirapakkul, W.; Tinchan, P.; Chaiseri, S. Effect of drying temperature on key odourants in kaffir lime (Citrus hystrix D.C., Rutaceae) leaves. Int. J. Food Sci. Technol. 2013, 48, 143–149. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Sawamura, M.; Tu, N.T.M.; Yu, X.; Xu, B. Volatile Constituents of the Peel Oils of Several Sweet Oranges in China. J. Essent. Oil Res. 2005, 17, 2–6. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, N.; Peng, Y.; Zhu, C.; Pan, S. Peel oils from three Citrus species: Volatile constituents, antioxidant activities and related contributions of individual components. J. Food Sci. Technol. 2019, 56, 4492–4502. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Yang, D.-J.; Liu, S.-C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Shim, J.-H.; Chae, J.-I.; Cho, S.-S. Identification and Extraction Optimization of Active Constituents in Citrus junos Seib ex TANAKA Peel and Its Biological Evaluation. Molecules 2019, 24, 680. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L. Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [Green Version]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Yang, W.-X.; Zhang, Q.; Zheng, Z.-A.; Wu, M.; Xiao, H.-W. Hot air impingement drying kinetics and quality attributes of orange peel. J. Food Process. Preserv. 2019, 44, e14294. [Google Scholar] [CrossRef]
- Liu, S.-C.; Tsai, C.-W. Effects of Heating Time on the Antioxidative Capacities of Citrus Fruit (Citrus sinensis (L.) Osbeck) By-products. Food Sci. Technol. Res. 2012, 18, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Kitts, D.D. Flavonoid composition of orange peel extract ameliorates alcohol-induced tight junction dysfunction in Caco-2 monolayer. Food Chem. Toxicol. 2017, 105, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Singh, B.; Gat, Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J. Food Sci. Technol. 2019, 56, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2010, 49, 276–282. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem. 2018, 270, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Sawalha, S.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE–MS/MS. Food Chem. 2009, 116, 567–574. [Google Scholar] [CrossRef]
- Chen, Z.-T.; Chu, H.-L.; Chyau, C.-C.; Chu, C.-C.; Duh, P.-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M.; Almusallam, I.A. Influence of drying techniques on bioactive properties, phenolic compounds and fatty acid compositions of dried lemon and orange peel powders. J. Food Sci. Technol. 2020, 58, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Omoba, O.S.; Obafaye, R.O.; Salawu, S.O.; Boligon, A.A.; Athayde, M.L. HPLC-DAD Phenolic Characterization and Antioxidant Activities of Ripe and Unripe Sweet Orange Peels. Antioxidants 2015, 4, 498–512. [Google Scholar] [CrossRef] [Green Version]
- Molina-Calle, M.; Priego-Capote, F.; de Castro, M.D.L. Development and application of a quantitative method for determination of flavonoids in orange peel: Influence of sample pretreatment on composition. Talanta 2015, 144, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Ho, S.-C. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010, 119, 868–873. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Cordoba, M.D.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Londoño-Londoño, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C.; Arango, G.J.; Pineda, J.R.R. Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Plaza-Oliver, M.; Beloqui, A.; Santander-Ortega, M.; Castro-Vázquez, L.; Rodríguez-Robledo, V.; Arroyo-Jiménez, M.; Préat, V.; Lozano, M. Ascorbyl-dipalmitate-stabilised nanoemulsions as a potential localised treatment of inflammatory bowel diseases. Int. J. Pharm. 2020, 586, 119533. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Kim, S.-Y.; Kim, D.-R.; Jo, S.-C.; Nam, K.C.; Ahn, A.D.U.; Lee, S.-C. Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and delivery of mandarin (Citrus reticulate Blanco) peel extracts by encapsulation of whey protein concentrate nanoparticles. LWT-Food Sci. Technol. 2019, 99, 24–33. [Google Scholar] [CrossRef]








| KI | COMPOUNDS | Navelina Peel | Salustriana Peel | Sanguina Peel | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Fresh | RSD | Mean FD | RSD | Mean Oven 50 °C | RSD | Mean Oven 70 °C | RSD | Mean Fresh | RSD | Mean FD | RSD | Mean Oven 50 °C | RSD | Mean Oven 70 °C | RSD | Mean Fresh | RSD | Mean FD | RSD | Mean Oven 50 °C | RSD | Mean Oven 70 °C | RSD | ||
| Sesquitenenes | |||||||||||||||||||||||||
| 1476 | α-copaene | 1.54 a | (7.76) | 1.71 a | (10.91) | 0.52 b | (8.32) | 0.38 b | (12.97) | 1.58 a | (14.91) | 1.2 a | (16.90) | 0.44 b | (0.59) | 0.34 c | (7.16) | 2.37 a | (2.13) | 2.92 b | (5.07) a | 1.26 c | (6.93) | 1.82 d | (5.75) |
| 1630 | γ-elemene | traces | 0.29 a | (11.78) | 0.26 a | (7.18) | 0.71 b | (7.52) | traces | 0.56 a | (8.13) | 0.36 b | (3.61) | 0.57 a | (5.61) | 0.33 a | (7.56) | 0.73 b | (8.15) | 0.13 c | (8.63) | 0.27 d | (10.80) | ||
| 1654 | β-farnesene | traces | 0.58 a | (9.82) | 0.24 b | (3.55) | 2.17 c | (15.98) | traces | 0.50 a | (0.77) | 0.25 b | (0.71) | 0.55 a | (16.94) | 0.27 a | (10.17) | 0.21 a | (5.82) | 0.24 a | (9.51) | 0.46 b | (13.48) | ||
| 1701 | valencene | 35.43 a | (0.98) | 36.67 a | (12.97) | 30.2 b | (7.82) | 13.31 c | (10.29) | 21.75 a | (5.06) | 27.45 b | (7.76) | 25.16 ab | (7.99) | 22.63 a | (7.99) | 20.54 a | (7.63) | 26.4 b | (9.38) | 5.86 c | (10.07) | 12.58 d | (8.63) |
| 1704 | germacrene D | 0.44 a | (0.00) | 0.81 b | (6.73) | 0.44 a | (5.08) | 0.12 c | (8.29) | 3.32 a | (20.20) | 3.37 a | (6.88) | 2.31 b | (12.91) | 2.04 b | (7.31) | 0.11 a | (6.15) | 0.38 b | (0.31) | 0.05 c | (1.36) | 0.21 d | (4.85) |
| 1723 | α-farnesene | 0.51 a | (0.34) | 0.87 b | (12.97) | 0.56 a | (0.28) | 0.42 c | (4.98) | 1.09 a | (0.29) | 0.88 b | (11.05) | 0.43 c | (7.88) | 0.31 d | (0.26) | 0.68 a | (0.98) | 3.22 b | (8.07) | 0.62 ac | (9.51) | 0.73 c | (10.16) |
| 1740 | δ-cadinene | traces | 0.5 a | (11.47) | 0.19 b | (6.86) | 0.62 a | (11.78) | traces | 0.36 a | (3.96) | 0.21 b | (4.56) | 0.26 b | (3.01) | 0.34 a | (5.66) | 0.91 b | (2.11) | 0.13 c | (10.97) | 0.65 d | (6.86) | ||
| 2016 | nerolidol | 2.06 a | (8.15) | 2.28 a | (11.55) | 0.42 b | (5.82) | 0.30 c | (6.86) | 0.99 a | (7.99) | 0.84 a | (3.91) | 0.64 c | (4.07) | 0.41 d | (5.35) | 0.66 a | (7.83) | 0.91 b | (6.82) | 0.41 c | (12.97) | 0.46 c | (2.77) |
| 2236 | β-sinensal | 11.8 a | (6.86) | 14.08 b | (7.44) | 5.69 c | (7.52) | 3.65 d | (7.52) | 3.19 a | (8.63) | 3.36 a | (2.00) | 2.6 b | (0.01) | 2.15 c | (0.28) | 5.17 a | (7.76) | 7.47 b | (5.70) | 0.35 c | (3.85) | 1.38 d | (0.98) |
| 2331 | α-sinensal | 10.95 a | (12.55) | 13.77 | (14.14) a | 4.91 b | (10.05) | 3.01 c | (10.05) | 4.32 a | (18.69) | 4.18 a | (18.21) | 1.86 b | (0.01) | 1.62 b | (11.39) | 4.32 a | (8.32) | 5.44 b | (3.33) | 0.73 c | (6.67) | 1.11 d | (8.07) |
| 2507 | nootkatone | 5.81 a | (7.82) | 6.16 a | (2.59) | 4.43 b | (7.56) | 2.86 b | (7.56) | 9.73 a | (1.22) | 11.03 b | (8.13) | 6.86 c | (0.78) | 4.03 d | (10.07) | 19.07 a | (5.70) | 27.75 b | (3.53) | 10.9 c | (9.59) | 13.21 d | (7.25) |
| 2083 | elemol | 3.93 a | (9.47) | 3.47 a | (9.38) | 0.81 a | (3.51) | 0.62 c | (4.98) | 1.82 a | (8.15) | 1.81 a | (13.48) | 1.81 a | (7.63) | 1.29 b | (7.63) | 1.3 a | (10.14) | 0.88 b | (8.13) | 1.65 c | (7.76) | 0.51 d | (0.77) |
| 2121 | γ-eudesmol | 1.12 a | (12.91) | 1.17 a | (3.55) | 0.25 b | (14.88) | 0.78 c | (7.52) | 0.67 a | (9.38) | 0.54 b | (6.67) | 0.51 b | (13.26) | 0.33 c | (13.26) | 0.21 a | (6.73) | 0.31 b | (0.01) | 0.32 b | (8.32) | 0.32 b | (4.07) |
| 2183 | δ-eudesmol | 2.15 a | (1.40) | 2.21 a | (9.38) | 1.17 b | (9.38) | 1.27 b | (2.28) | 5.05 a | (10.05) | 9.09 b | (7.88) | 5.09 a | (6.67) | 3.83 c | (6.67) | 7.81 a | (1.75) | 9.51 b | (2.77) | 4.68 c | (13.26) | 7.57 a | (7.16) |
| 2348 | (E)-farnesol | 1.33 a | (7.63) | 1.93 b | (3.63) | 1.4 a | (8.63) | 1.03 c | (8.07) | 1.58 a | (12.55) | 1.24 ab | (4.85) | 1.08 b | (0.01) | 0.68 c | (0.01) | 1.04 a | (7.52) | 1.62 b | (7.31) | 0.97 a | (12.49) | 1.02 a | (6.86) |
| Total | 76.57 | 86.5 | 51.49 | 31.25 | 55.09 | 66.41 | 49.61 | 41.04 | 64.22 | 88.66 | 28.3 | 42.3 | |||||||||||||
| Terpene oxide | |||||||||||||||||||||||||
| 1428 | (Z)-linalool oxide | 0.11 a | (7.83) | 0.21 b | (6.07) | 0.19 b | (4.47) | traces | 0.17 a | (8.44) | 0.44 b | (8.63) | 0.78 c | (5.14) | 0.51 b | (6.36) | 0.07 a | (10.88) | 0.60 b | (7.75) | 0.57 b | (3.40) | traces | ||
| 1430 | (Z)-limonene-oxide | 0.31 a | (4.98) | 0.64 b | (3.82) | 0.74 c | (0.77) | traces | 0.11 a | (6.73) | 0.79 b | (2.70) | 0.91 c | (7.63) | 0.42 c | (7.04) | 0.09 a | (8.32) | 0.32 b | (6.86) | 0.41 b | (5.82) | 0.20 c | (7.91) | |
| 1453 | (E)-linalool oxide | 0.16 a | (1.40) | 0.65 b | (6.87) | 1.24 c | (2.77) | traces | 0.25 a | (22.63) | 0.29 ab | (15.48) | 0.31 b | (6.96) | 0.01 c | (0.00) | 0.10 a | (14.14) | 0.27 b | (8.63) | 0.67 c | (5.08) | 0.18 ab | (6.82) | |
| Total | 0.58 | 1.50 | 2.17 | 0.03 | 0.53 | 1.52 | 2.00 | 0.94 | 0.26 | 1.19 | 1.65 | 0.39 | |||||||||||||
| Terpenoids alcohols | |||||||||||||||||||||||||
| 1541 | linalool | 66.79 a | (0.88) | 79.79 b | (7.86) | 81.02 b | (6.33) | 48.74 c | (2.24) | 32.52 a | (7.44) | 35.17 a | (14.76) | 45.12 b | (1.53) | 18.47 c | (6.90) | 63.90 a | (6.67) | 108.33 b | (3.24) | 80.96 | (1.36) | 24.52 | (6.99) |
| 1585 | α-terpineol | 32.08 a | (11.55) | 25.01 b | (7.33) | 7.81 b | (7.25) | 7.78 b | (7.44) | 17.61 a | (8.71) | 20.42 b | (7.91) | 7.61 c | (6.86) | 12.01 d | (6.86) | 34.72 a | (12.91) | 29.19 b | (7.52) | 7.00 | (7.99) | 5.86 | (1.13) |
| 1605 | p-menthadienol (I) | 7.35 a | (8.07) | 6.36 b | (5.14) | 13.13 b | (12.91) | 5.19 c | (4.98) | 5.62 a | (11.55) | 5.71 a | (7.75) | 7.76 c | (11.55) | 4.45 d | (7.63) | 8.26 a | (6.09) | 9.76 b | (8.82) | 11.41 | (4.02) | 2.37 | (10.29) |
| 1611 | p-menth-2-en-1-ol | 0.33 a | (14.98) | 0.35 a | (8.15) | 0.21 b | (13.64) | 0.69 c | (11.78) | 0.47 a | (7.88) | 0.37 b | (8.44) | 0.36 b | (4.98) | 0.37 b | (4.98) | 0.25 a | (7.82) | 0.36 b | (7.63) | 0.27 a | (8.57) | 0.09 | (8.32) |
| 1646 | p-menthadienol (II) | 8.94 a | (5.47) | 7.12 b | (7.63) | 7.70 b | (1.40) | 3.54 c | (12.97) | 4.90 a | (7.63) | 4.97 a | (1.16) | 4.12 b | (2.63) | 3.43 c | (8.44) | 6.08 a | (7.88) | 5.90 a | (9.68) | 5.62 ab | (7.63) | 5.19 b | (11.91) |
| 1667 | β-citronellol | 9.61 a | (2.52) | 8.66 b | (3.84) | 12.26 b | (7.44) | 7.64 c | (2.52) | 8.37 a | (0.75) | 8.26 a | (7.82) | 7.85 b | (0.75) | 6.41 c | (9.38) | 2.31 a | (7.56) | 2.77 a | (6.82) | 1.90 b | (9.85) | 5.13 c | (13.38) |
| 1786 | nerol | 14.48 a | (5.81) | 15.60 a | (8.63) | 10.26 b | (4.90) | 12.58 c | (7.63) | 7.46 a | (5.60) | 7.53 a | (7.04) | 7.99 b | (8.60) | 2.20 c | (13.45) | 5.78 a | (13.15) | 5.72 a | (4.07) | 1.38 b | (5.05) | 0.40 c | (7.31) |
| 1838 | geraniol | 14.46 a | (13.24) | 17.84 b | (7.56) | 7.13 b | (8.31) | 0.03 c | (0.00) | 8.97 a | (10.03) | 8.99 a | (7.52) | 5.88 b | (10.03) | 3.39 c | (15.98) | 8.60 a | (9.51) | 8.25 a | (13.26) | 1.46 b | (3.78) | 0.65 c | (5.70) |
| 1834 | (Z)-carveol | 2.28 a | (10.54) | 2.51 a | (4.13) | 2.42 a | (12.63) | 0.03 c | (0.00) | 2.77 a | (3.02) | 4.77 b | (1.94) | 1.53 c | (2.75) | 1.64 c | (7.56) | 1.10 a | (3.33) | 2.05 b | (3.85) | 1.70 c | (1.05) | 0.91 d | (1.91) |
| 1927 | limonyl alcohol | 0.73 a | (8.15) | 0.75 a | (8.07) | 0.59 b | (8.07) | 0.61 b | (7.63) | 0.58 a | (0.00) | 0.82 b | (2.25) | 0.62 a | (3.45) | 0.37 c | (6.99) | 0.21 a | (5.15) | 0.38 b | (4.67) | 0.20 a | (3.05) | 0.12 c | (6.86) |
| 1963 | p-mentha-dien-9-ol | 1.32 a | (9.51) | 1.73 ab | (9.31) | 1.98 b | (6.99) | 1.25 b | (8.63) | 3.58 a | (3.43) | 3.71 a | (6.50) | 2.64 b | (5.43) | 2.36 b | (5.82) | 0.63 a | (5.05) | 1.09 b | (5.14) | 0.61 a | (13.73) | 0.40 c | (7.52) |
| 1996 | perillyl alcohol | 2.04 a | (7.18) | 2.14 a | (0.03) | 2.14 a | (14.74) | 0.81 c | (6.86) | 1.34 a | (2.42) | 2.34 b | (7.52) | 0.79 c | (1.38) | 0.45 d | (9.51) | 0.49 a | (9.59) | 2.76 b | (8.44) | 0.70 c | (2.37) | 0.30 d | (2.87) |
| 2140 | eugenol | 0.39 a | (8.15) | 0.52 b | (3.61) | 0.38 a | (10.05) | 0.16 c | (8.15) | 1.03 a | (3.85) | 1.75 b | (7.52) | 0.98 a | (3.85) | 0.07 c | (5.70) | 0.27 a | (7.31) | 0.34 b | (7.12) | 0.25 a | (7.63) | 0.10 c | (12.97) |
| Total | 160.2 | 168.41 | 147.09 | 88.85 | 95.42 | 104.02 | 93.45 | 55.62 | 132.82 | 176.4 | 113.48 | 46.04 | |||||||||||||
| Monoterpenoid aldehydes | |||||||||||||||||||||||||
| 1468 | citronellal | 3.58 a | (6.33) | 3.99 a | (10.40) | 2.57 b | (7.76) | 0.00 c | 1.14 a | (5.82) | 1.87 b | (4.07) | 1.70 c | (0.71) | 0.00 d | 2.81 a | (0.60) | 3.19 b | (3.35) | 0.99 c | (8.82) | 0.00 d | |||
| 1664 | neral | 3.66 a | (4.95) | 2.88 b | (2.97) | 4.74 c | (1.26) | 7.15 d | (6.73) | 1.33 a | (3.30) | 2.84 b | (6.90) | 3.37 c | (7.45) | 4.11 d | (10.14) | 2.20 a | (2.16) | 4.29 b | (2.67) | 5.95 c | (9.51) | 9.94 d | (10.29) |
| 1712 | geranial | 5.61 a | (1.55) | 4.66 b | (6.73) | 3.74 c | (6.87) | 2.26 d | (5.82) | 3.19 a | (5.99) | 2.09 b | (7.63) | 1.37 c | (9.33) | 1.56 c | (7.63) | 3.60 a | (14.24) | 4.19 b | (11.41) | 7.83 c | (19.37) | 5.69 d | (0.02) |
| Total | 12.85 | 11.53 | 11.05 | 9.41 | 5.66 | 6.8 | 6.44 | 5.87 | 8.61 | 11.67 | 14.77 | 15.63 | |||||||||||||
| Chain aldehydes | |||||||||||||||||||||||||
| 1284 | octanal | 12.04 a | (6.73) | 9.98 b | (1.51) | 5.08 | (7.16) | 3.39 | (5.97) | 6.98 a | (8.38) | 7.17 a | (17.05) | 5.84 c | (7.31) | 2.77 d | (9.94) | 9.57 a | (14.76) | 8.70 b | (1.00) | 7.04 c | (2.00) | 2.33 d | |
| 1386 | nonanal | 2.58 a | (8.13) | 1.63 b | (6.09) | 1.52 b | (13.26) | 0.73 c | (7.56) | 2.00 a | (1.01) | 1.65 b | (3.19) | 1.88 ab | (4.86) | 1.15 c | (2.42) | 2.01 a | (7.63) | 2.10 a | (3.61) | 2.54 b | (2.16) | 0.05 c | |
| 1490 | decanal | 13.91 a | (7.31) | 12.22 b | (4.14) | 11.00 c | (6.09) | 7.00 d | (8.32) | 11.52 a | (3.31) | 11.00 a | (5.08) | 5.86 b | (1.02) | 3.86 c | (1.55) | 4.76 a | (7.63) | 3.44 b | (7.56) | 3.21 b | (5.98) | 0.34 c | |
| Total | 27.83 | 23.93 | 18.1 | 11.12 | 20.5 | 20.42 | 13.58 | 7.78 | 16.34 | 14.24 | 12.49 | 2.72 | |||||||||||||
| Terpene hydrocarbons | |||||||||||||||||||||||||
| 1018 | α- pinene | 13.49 a | (2.77) | 13.58 a | (1.48) | 21.97 b | (4.98) | 8.48 c | (8.03) | 11.12 a | (7.75) | 19.76 b | (3.31) | 21.85 c | (0.98) | 9.50 d | (0.98) | 17.57 a | (4.60) | 17.85 a | (3.05) | 11.58 c | (3.00) | 10.21 d | (10.81) |
| 1104 | β- pinene | 5.63 a | (4.52) | 6.36 b | (5.79) | 7.51 c | (2.76) | 5.20 d | (11.55) | 4.60 a | (8.13) | 5.80 b | (7.91) | 7.43 c | (7.18) | 2.00 d | (8.13) | 11.34 a | (7.56) | 9.64 b | (7.82) | 13.34 c | (11.22) | 1.61 d | (7.76) |
| 1119 | sabinene | 7.65 a | (13.34) | 9.47 b | (2.17) | 13.94 c | (8.32) | 4.84 d | (4.98) | 5.90 a | (10.07) | 7.14 b | (2.87) | 10.53 c | (7.99) | 3.10 d | (2.87) | 9.56 a | (3.97) | 11.84 b | (4.13) | 14.25 c | (6.73) | 3.69 d | (8.63) |
| 1209 | limonene | 1687.15 a | (4.98) | 1637.25 a | (1.59) | 2055.82 b | (7.31) | 986.05 c | (11.78) | 1677.04 | a (0.79) | 1700.36 b | (0.65) | 2005.28 c | (0.44) | 852.33 d | (7.83) | 2107.74 a | (3.65) | 2190.26 b | (0.72) | 2424.57 c | (8.32) | 925.92 d | (7.31) |
| 1237 | (Z)-b-ocimene | 0.31 a | (11.78) | 0.48 b | (7.16) | 0.75 c | (1.54) | 0.15 d | (3.61) | 0.23 a | (7.99) | 0.32 b | (0.77) | 0.43 c | (12.91) | 0.14 d | (0.77) | 0.45 a | (10.40) | 0.41 a | (6.90) | 0.59 b | (7.63) | 0.00 c | |
| 1254 | γ-terpinene | 3.19 a | (5.70) | 3.46 b | (5.95) | 3.77 c | (3.26) | 2.21 d | (1.40) | 2.08 a | (9.45) | 2.34 a | (8.47) | 3.37 c | (10.29) | 2.21 d | (7.45) | 3.14 a | (0.71) | 3.99 b | (1.98) | 2.72 c | (7.52) | 0.78 d | (10.92) |
| 1256 | (E) b-ocimene | 1.73 a | (3.33) | 2.28 b | (14.80) | 3.26 c | (1.40) | 1.59 d | (9.82) | 1.39 a | (8.71) | 1.69 a | (9.82) | 3.23 c | (7.12) | 0.76 d | (2.78) | 0.60 a | (1.94) | 0.49 b | (6.90) | 0.64 a | (0.26) | 0.41 b | (5.69) |
| 1269 | p-cymene | 0.73 a | (4.11) | 0.86b | (4.79) | 0.91 b | (3.51) | 0.69 c | (12.97) | 0.42 a | (9.04) | 0.46 a | (11.17) | 0.46 a | (9.61) | 0.20 d | (2.77) | 0.38 a | (3.31) | 0.40 a | (3.61) | 1.09 b | (7.25) | 0.25 c | (6.86) |
| 1289 | α-terpinolene | 2.55 a | (8.38) | 2.47 a | (10.14) | 3.01 b | (5.14) | 1.21 c | (12.32) | 2.50 a | (6.93) | 2.36 a | (8.22) | 3.28 b | (8.32) | 1.42 c | (8.32) | 3.83 a | (7.99) | 4.42 b | (7.44) | 3.15 c | (9.07) | 0.59 d | (8.62) |
| 1368 | alloocimene | 0.11 a | (8.13) | 0.11 a | (1.75) | 0.20 b | (17.91) | 0.10 c | (0.98) | 0.17 a | (3.55) | 0.15 a | (7.41) | 0.18 a | (14.93) | 0.07 d | (2.70) | 0.09 a | (8.47) | 0.12 a | (7.82) | 0.23 c | (3.13) | 0.08 a | (11.55) |
| 1427 | p-cymenene | 0.31 a | (7.99) | 0.66 | (6.86) | 1.68 b | (2.87) | 0.75 c | (7.41) | 0.23 a | (7.44) | 0.10 b | (7.44) | 0.62 c | (9.05) | 0.00 d | 0.35 a | (7.88) | 1.35 b | (6.40) | 1.48 b | (5.70) | 0.19 c | (3.61) | |
| 1521 | β-cubenene | 0.35 a | (7.52) | 0.69 b | (6.73) | 0.90 c | (7.16) | 0.42 d | (7.31) | 1.23 a | (3.33) | 0.54 b | (3.33) | 0.83 c | (7.63) | 1.28 a | (7.18) | 0.44 a | (13.26) | 0.41 a | (6.09) | 1.20 c | (6.93) | 0.28 d | (8.15) |
| 1578 | transCaryophyllene | 2.14 a | (6.86) | 2.96 b | (5.82) | 6.62 b | (2.59) | 1.77 c | (6.86) | 4.09 a | (11.62) | 4.45 ab | (4.60) | 4.77 b | (1.36) | 2.01 c | (6.09) | 2.75 a | (8.13) | 3.59 b | (1.57) | 7.50 c | (10.89) | 2.14 d | (9.38) |
| 1703 | β-selinene | 2.52 a | (0.36) | 3.30 b | (1.49) | 4.43 c | (7.21) | 0.30 d | (8.15) | 4.58 a | (10.72) | 4.13 a | (11.69) | 5.81 b | (6.44) | 2.39 c | (14.76) | 2.30 a | (3.26) | 2.90 b | (7.52) | 4.67 c | (4.65) | 1.62 d | (7.88) |
| Total | 1727.86 | 1683.49 | 2124.68 | 1013.76 | 1715.58 | 1749.1 | 2068.09 | 877.72 | 2190.64 | 2236.98 | 2487.17 | 947.84 | |||||||||||||
| Terpene Esters | |||||||||||||||||||||||||
| 1546 | linalyl acetate | 0.82 a | (6.86) | 0.64 b | (0.22) | 0.41 c | (8.32) | 0.00 d | 0.09 a | (12.91) | 0.11 a | (10.00) | 0.01 b | (1.00) | 0.00 c | 1.13 a | (13.65) | 0.89 b | (5.06) | 0.41 c | (4.98) | 0.29 d | (1.01) | ||
| 1570 | bornyl acetate | 0.48 a | (8.63) | 0.26 b | (6.99) | 0.24 b | (5.70) | 0.00 c | 0.38 a | (1.09) | 0.36 b | (6.53) | 0.34 a | (1.02) | 0.12 b | (14.76) | 0.19 a | (1.82) | 0.25 b | (1.00) | 0.01 c | (0.00) | 0.01 d | (0.00) | |
| 1644 | citronelly acetate | 0.23 a | (6.86) | 0.20 a | (10.05) | 0.12 b | (3.61) | 0.11 b | (9.82) | 0.16 a | (1.40) | 0.14 b | (1.40) | 0.00 b | 0.00 b | 1.41 a | (5.70) | 1.33 a | (6.99) | 1.41 a | (8.44) | 0.68 b | (2.15) | ||
| 1673 | terpinyl acetate | 3.80 a | (13.65) | 3.49 a | (13.26) | 1.08 b | (10.14) | 0.82 c | (12.97) | 1.36 a | (0.00) | 1.30 a | (0.00) | 1.16 b | (7.12) | 0.64 c | (6.73) | 3.22 a | (5.70) | 2.51 b | (2.63) | 0.45 c | (8.47) | 0.57 d | (8.13) |
| 1711 | geranyl acetate | 0.51 a | (4.30) | 0.49 a | (6.86) | 0.30 b | (10.14) | 0.17 c | (7.63) | 1.38 a | (7.42) | 0.49 b | (5.70) | 0.37 c | (8.71) | 0.27 d | (11.92) | 0.53 a | (10.07) | 0.55 a | (6.86) | 0.51 a | (7.75) | 0.26 b | (7.82) |
| 1742 | neryl acetate | 0.72 a | (12.55) | 0.77 a | (3.61) | 0.39 b | (3.64) | 0.23 c | (8.15) | 0.68 a | (2.46) | 0.66 a | (2.68) | 0.50 b | (0.00) | 0.32 c | (10.68) | 1.04 a | (3.92) | 1.09 a | (1.82) | 0.80 b | (2.90) | 0.34 d | (5.09) |
| Total | 6.56 | 5.72 | 2.54 | 1.39 | 4.05 | 3.08 | 2.39 | 1.36 | 7.72 | 6.38 | 3.59 | 2.15 | |||||||||||||
| C6 Alcohols | |||||||||||||||||||||||||
| 1350 | 1-hexanol | 10.21 a | (12.97) | 9.83 a | (0.31) | 1.04 b | (8.13) | 0.00 c | 2.76 a | (1.01) | 2.01 b | (1.36) | 0.76 c | (36.35) | 0.00 d | 1.73 a | (6.86) | 1.69 a | (9.68) | 0.67 b | (12.91) | 0.32 c | (8.63) | ||
| 1381 | (Z)-3-hexen-1-ol | 3.55 a | (0.98) | 3.39 a | (2.08) | 0.26 b | (10.07) | 0.00 c | 1.30 a | (8.19) | 1.07 a | (5.98) | 0.19 b | (0.00) | 0.00 c | 0.58 a | (8.71) | 0.52 a | (8.63) | 0.14 b | (4.98) | 0.00 c | |||
| Total | 13.76 | 13.22 | 1.30 | 0,00 | 4.06 | 3.08 | 0.95 | 0.00 | 2.31 | 2.11 | 0.81 | 0.33 | |||||||||||||
| Phenolic Acids | Navelina Peel (Citrus sinensis L. Osbeck cv. “Navelina”) | Salustriana Peel (Citrus sinensis L. Osbeck cv. “Salustriana”) | Sanguina Peel (Citrus x sinensis var. “Sanguina”) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | RSD | OD 50 °C | RSD | OD 70 °C | RSD | FD | RSD | Fresh | RSD | OD 50 °C | RSD | OD 70 °C | RSD | FD | RSD | Fresh | RSD | OD 50 °C | RSD | OD 70 °C | RSD | FD | RSD | |
| Gallic acid | 0.03 a | (2.40) | 0.03 a | (10.10) | 0.14 b | (6.24) | 0.29 c | (2.40) | 0.03 a | (10.48) | 0.04 a | (12.41) | 0.13 b | (9.88) | 0.29 c | (4.88) | 0.03 a | (6.73) | 0.03 a | (2.40) | 0.15 b | (3.87) | 0.40 c | (3.45) |
| p-Hydroxybenzoic acid | 0.02 a | (7.79) | 0.03 a | (9.43) | 0.07 b | (8.88) | 0.10 c | (7.44) | 0.02 a | (11.49) | 0.03 a | (10.48) | 0.06 b | (7.95) | 0.09 c | (13.70) | 0.05 a | (11.79) | 0.06 a | (9.43) | 0.14 b | (7.88) | 0.20 c | (7.44) |
| Vanillic acid | 0.07 a | (6.24) | 0.07 | (1.00) | 0.26 b | (8.32) | 0.73 c | (7.75) | 0.06 a | (9.99) | 0.06 a | (14.08) | 0.22 b | (8.64) | 0.64 c | (4.65) | 0.14 a | (6.24) | 0.15 a | (1.00) | 0.54 b | (8.32) | 1.54 c | (7.75) |
| Chlorogenic acid | 0.17 a | (8.32) | 0.19 a | (7.44) | 1.08 b | (7.24) | 1.94 c | (5.48) | 0.14 a | (12.31) | 0.17 a | (10.64) | 0.93 b | (7.83) | 1.67 c | (6.35) | 0.36 a | (8.32) | 0.40 a | (7.44) | 2.27 b | (7.24) | 4.08 c | (5.48) |
| Caffeic acid | 0.30 a | (4.71) | 0.38 a | (1.75) | 0.75 b | (8.54) | 1.02 c | (10.45) | 0.26 a | (4.54) | 0.31 a | (4.68) | 0.65 b | (6.46) | 0.90 c | (6.09) | 0.63 a | (4.71) | 0.85 a | (1.75) | 1.57 b | (8.54) | 2.14 c | (10.45) |
| p-Coumaric acid | 0.31 a | (8.40) | 0.38 a | (1.89) | 0.43 b | (3.29) | 0.89 c | (3.99) | 0.27 a | (9.92) | 0.29 a | (14.63) | 0.38 b | (5.42) | 0.75 c | (6.88) | 0.66 a | (8.40) | 0.67 a | (0.89) | 0.91 b | (3.29) | 1.87 c | (3.99) |
| Ferulic acid | 0.45 a | (2.37) | 0.46 a | (0.77) | 1.36 b | (1.57) | 1.38 c | (4.63) | 0.38 a | (5.89) | 0.38 a | (4.73) | 1.17 b | (2.27) | 1.22 c | (3.39) | 0.94 a | (2.37) | 0.97 a | (0.77) | 2.86 b | (1.57) | 2.90 c | (4.63) |
| Sinapic acid | 0.40 a | (3.54) | 0.41 a | (8.73) | 1.0 5 b | (8.80) | 1.62 c | (8.32) | 0.35 a | (5.00) a | 0.35 a | (10.64) | 0.91 b | (9.92) | 1.36 c | (5.35) | 0.84 a | (3.54) | 0.85 a | (8.73) | 2.20 b | (8.80) | 3.41 c | (8.32) |
| Total acids | 1.75 a | 1.94 a | 5.11 b | 7.94 c | 1.50 a | 1.63 a | 4.44 b | 6.92 c | 3.66 a | 3.98 a | 10.63 b | 16.54 c | ||||||||||||
| Tentative Identification | Retention Time (min) | UVmax (nm) | MS [M − H]− (m/z) | MS [M + H]+ (m/z) | Products Ions (m/z) |
|---|---|---|---|---|---|
| Rutin [89] | 11.43 | 256, 286, 351 | 611 | 300.8, 342.8 | |
| Narirutin [87,90] | 13.58 | 217, 284, 331 | 579 | 271, 151 | |
| Naringin [87,90] | 18.02 | 224, 283,331 | 579 | 459, 271 | |
| Hesperidin [87,90] | 20.19 | 225, 284, 328 | 609 | 301, 198 | |
| Naringenin [87,89,90] | 24.65 | 226, 284, 325 | 273 | 153 | |
| Hesperetin [89] | 26.57 | 225, 285, 329 | 303 | 285 | |
| Sinensetin [89,90] | 28.04 | 243, 264, 333 | 373 | 358, 343, 312 | |
| Quercetogenin [89] | 31.98 | 250, 272, 335 | 403 | 388, 373 | |
| Nobiletin [89,90] | 35.46 | 248, 268, 334 | 403 | 388, 373 | |
| Tangeretin [87,89,90] | 36.54 | 271, 322 | 373 | 358, 343, 325, 297 |
| Navelina Peel (Citrus sinensis L. Osbeck cv. “Navelina”) | Salustriana Peel (Citrus sinensis L. Osbeck cv. “Salustriana”) | Sanguina Peel (Citrus x sinensis var. “Sanguina”) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | |
| Flavonoids Rutin | 3.24 a | (0.24) | 2.62 b | (9.87) | 1.55 c | (0.45) | 3.11 a | (1.81) | 3.32 a | (2.21) | 2.62 b | (1.62) | 1.11 c | (12.49) | 2.98 a | (2.98) | 5.92 a | (3.21) | 3.94 b | (2.51) | 2.47 c | (7.18) | 5.37 a | (2.98) |
| Narirutin | 28.10 a | (3.11) | 21.56 b | (4.36) | 18.44 c | (11.92) | 27.87 a | (2.51) | 26.87 a | (3.25) | 19.61 b | (0.35) | 17.01 c | (7.75) | 23.92 d | (1.57) | 47.83 a | (3.25) | 39.18 b | (0.66) | 31.38 c | (2.53) | 42.57 d | (1.57) |
| Hesperidin | 124.73 a | (3.63) | 92.97 b | (4.21) | 84.22 c | (0.55) | 113.70 d | (2.73) | 124.00 a | (3.65) | 92.06 b | (0.52) | 92.23 c | (4.24) | 114.97 d | (2.75) | 202.32 a | (3.65) | 165.21 b | (3.81) | 135.49 c | (0.51) | 187.59 d | (2.75) |
| Neohesperidin | 11.62 a | (1.30) | 8.53 b | (3.84) | 7.02 c | (0.26) | 10.90 d | (0.03) | 10.39 a | (1.45) | 7.80 b | (4.87) | 6.79 c | (0.27) | 9.67 a | (5.03) | 18.49 a | (1.45) | 15.49 b | (0.80) | 13.87 c | (0.24) | 17.72 a | (9.03) |
| Naringin | 12.15 a | (2.08) | 7.25 b | (0.12) | 5.80 c | (1.35) | 10.40 d | (2.84) | 10.92 a | (2.32) | 6.57 b | (1.19) | 5.02 c | (0.18) | 10.14 a | (3.22) | 19.44 a | (2.32) | 12.70 b | (1.10) | 10.71 c | (0.15) | 16.32 d | (3.22) |
| Hesperetin | 5.55 a | (0.29) | 6.98 b | (0.93) | 8.87 c | (0.72) | 8.05 d | (2.76) | 4.61 a | (0.15) | 5.04 b | (1.19) | 7.53 c | (6.39) | 6.09 d | (2.91) | 8.21 a | (0.15) | 10.09 b | (0.78) | 12.51 c | (3.22) | 11.20 d | (2.27) |
| Naringenin | 3.77 a | (2.63) | 4.73 b | (5.54) | 7.42 c | (0.10) | 6.13 d | (2.08) | 3.64 a | (2.32) | 4.19 b | (0.62) | 6.93 c | (0.71) | 5.19 d | (1.70) | 3.57 a | (0.15) | 4.90 b | (0.87) | 6.48 c | (2.32) | 5.44 d | (3.22) |
| Sinensetin | 1.64 a | (4.94) | 1.61 a | (0.60) | 2.28 c | (4.44) | 2.05 d | (0.58) | 0.41 a | (12.66) | 0.38 b | (2.53) | 0.95 c | (3.87) | 0.82 d | (0.70) | 0.73 a | (11.66) | 0.69 a | (2.53) | 1.69 c | (3.87) | 1.46 d | (0.70) |
| Quercetogenin | 2.79 a | (3.47) | 3.35 b | (5.04) | 4.98 c | (0.14) | 4.26 d | (2.03) | 2.06 a | (12.56) | 3.02 b | (1.87) | 4.18 c | (6.12) | 3.70 d | (2.43) | 3.67 a | (12.56) | 3.04 a | (2.43) | 7.11 c | (0.17) | 6.36 d | (2.42) |
| Nobiletin | 8.53 a | (3.84) | 9.02 b | (0.21) | 11.91 c | (2.17) | 10.90 d | (0.03) | 7.95 a | (11.13) | 9.28 b | (0.84) | 12.83 c | (5.82) | 11.17 d | (2.64) | 16.51 a | (0.84) | 15.46 a | (1.90) | 26.40 c | (5.82) | 19.88 d | (2.64) |
| Tangeretin | 3.37 a | (1.54) | 4.23 b | (6.63) | 6.34 c | (2.48) | 5.53 d | (2.15) | 3.60 a | (0.32) | 4.00 b | (1.88) | 6.12 c | (4.85) | 5.04 d | (5.20) | 6.41 a | (0.32) | 7.12 b | (1.88) | 10.89 c | (4.85) | 8.96 d | (5.20) |
| TPC (mg GAE·g−1 DW) | 22.75 a | (10.88) | 30.50 b | (6.96) | 42.00 c | (10.10) | 53.29 d | (1.09) | 19.55 a | (3.26) | 27.16 b | (1.87) | 31.27 c | (10.27) | 45.09 d | (3.42) | 41.66 a | (1.17) | 64.36 b | (6.96) | 83.15 c | (1.45) | 117.15 d | (4.64) |
| TFC mg (QE·g−1 DW) | 12.64 a | (1.23) | 16.94 b | (1.04) | 23.33 c | (4.13) | 29.61 d | (6.96) | 10.86 a | (9.88) | 15.09 b | (3.12) | 19.37 c | (4.43) | 25.05 d | (7.25) | 23.14 a | (5.77) | 30.7 5 b | (3.45) | 35.19 c | (1.86) | 46.00 d | (0.44) |
| Navelina Peel (Citrus sinensis L. Osbeck cv. “Navelina”) | Salustriana Peel (Citrus sinensis L. Osbeck cv. “Salustriana”) | Sanguina Peel (Citrus x sinensis var. “Sanguina”) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | Fresh | RSD | Oven 50 °C | RSD | Oven 70 °C | RSD | FD | RSD | |
| DPPH | 44.82 a | (11.94) | 56.30 b | (2.54) | 64.96 c | (4.43) | 71.57 d | (0.84) | 33.62 a | (11.94) | 42.22 b | (2.54) | 47.72 c | (4.52) | 53.68 d | (0.84) | 67.24 a | (11.94) | 80.45 b | (0.95) | 93.38 c | (1.53) | 107.36 d | (0.84) |
| FRAP | 26.89 a | (11.94) | 33.78 b | (2.54) | 42.92 c | (3.58) | 49.49 d | (6.16) | 18.83 a | (11.94) | 23.65 b | (2.54) | 30.04 c | (3.58) | 34.64 d | (6.16) | 38.73 a | (11.94) | 48.64 b | (2.54) | 63.36 c | (0.01) | 71.26 d | (6.16) |
| ABTS | 144.94 a | (2.44) | 213.62 b | (2.65) | 253.35 c | (1.95) | 313.07 d | (0.43) | 122.48 a | (0.56) | 159.08 b | (1.87) | 196.09 c | (0.01) | 239.85 d | (3.23) | 202.69 a | (0.61) | 283.48 b | (0.97) | 364.58 c | (5.54) | 569.23 d | (0.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Vázquez, L.; Lozano, M.V.; Rodríguez-Robledo, V.; González-Fuentes, J.; Marcos, P.; Villaseca, N.; Arroyo-Jiménez, M.M.; Santander-Ortega, M.J. Pressurized Extraction as an Opportunity to Recover Antioxidants from Orange Peels: Heat treatment and Nanoemulsion Design for Modulating Oxidative Stress. Molecules 2021, 26, 5928. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195928
Castro-Vázquez L, Lozano MV, Rodríguez-Robledo V, González-Fuentes J, Marcos P, Villaseca N, Arroyo-Jiménez MM, Santander-Ortega MJ. Pressurized Extraction as an Opportunity to Recover Antioxidants from Orange Peels: Heat treatment and Nanoemulsion Design for Modulating Oxidative Stress. Molecules. 2021; 26(19):5928. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195928
Chicago/Turabian StyleCastro-Vázquez, Lucía, María Victoria Lozano, Virginia Rodríguez-Robledo, Joaquín González-Fuentes, Pilar Marcos, Noemí Villaseca, Maria Mar Arroyo-Jiménez, and Manuel J. Santander-Ortega. 2021. "Pressurized Extraction as an Opportunity to Recover Antioxidants from Orange Peels: Heat treatment and Nanoemulsion Design for Modulating Oxidative Stress" Molecules 26, no. 19: 5928. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195928