Construction of Two Stable Co(II)-Based Hydrogen-Bonded Organic Frameworks as a Luminescent Probe for Recognition of Fe3+ and Cr2O72− in H2O
Abstract
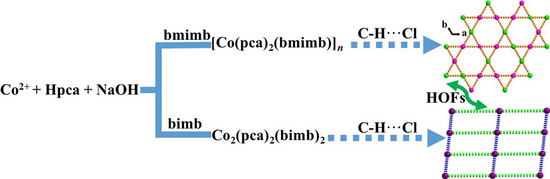
:1. Introduction
2. Results and Discussion
2.1. Description of the Structure of [Co(pca)2(bmimb)]n (1)
2.2. Description of the Structure of [Co2(pca)4(bimb)2] (2)
2.3. Luminescent Properties
2.3.1. Solid State Fluorescence Properties of 1 and 2
2.3.2. Detection of Fe3+ Ion
2.3.3. Detection of Cr2O72− Anions
2.3.4. Stability and Cyclic Use Test
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of [Co(pca)2(bmimb)]n (1)
3.4. Synthesis of [Co2(pca)4(bimb)2] (2)
3.5. Comparison of Synthesis and Structure for 1 and 2
3.6. Photoluminescent Sensing Experiments
3.7. Fluorescence Titration Experiments for Fe3+ and Cr2O72−
3.8. Recyclable Luminescence Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Karmakar, A.; Illathvalappil, R.; Anothumakkool, B.; Sen, A.; Samanta, P.; Desai, A.V.; Kurungot, S.; Ghosh, S.K. Hydrogen-Bonded Organic Frameworks (HOFs): A New Class of Porous Crystalline Proton-Conducting Materials. Angew. Chem. Int. Ed. 2016, 55, 10667–10671. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shi, H.; Zhang, Z.; Wang, X.; Ma, H.; Gan, N.; Wu, Q.; Cheng, Z.; Ling, K.; Gu, M.; et al. Hydrogen-Bonded Organic Aromatic Frameworks for Ultralong Phosphorescence by Intralayer π−π Interactions. Angew. Chem. Int. Ed. 2018, 57, 4005–4009. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, J.W.; Zhang, J.H.; Lai, S.; Zhong, D.C. Hydrogen-bonded organic frameworks: Design, structures and potential applications. CrystEngComm 2018, 20, 5884–5898. [Google Scholar] [CrossRef]
- Han, Y.F.; Yuan, Y.X.; Wang, H.B. Porous Hydrogen-Bonded Organic Frameworks. Molecules 2017, 22, 266. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Lin, R.B.; Zhang, Z.; Xiang, S.; Chen, B. Hydrogen-Bonded Organic Frameworks as a Tunable Platform for Functional Materials. J. Am. Chem. Soc. 2020, 142, 14399–14416. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Yu, J.; Cui, Y.; Yang, Y.; Wang, Z.; Yang, D.; Qian, G. Luminescent metal-organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef]
- Taylor, K.M.; Rieter, W.J.; Lin, W. Manganese-Based Nanoscale Metal-Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 14358–14359. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.; Della, R.J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal–organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.S.; Guo, S.P.; Li, Y.; Cai, L.Z.; Zou, J.-P.; Xu, G.; Zhou, W.-W.; Zheng, F.-K.; Guo, G.-C. A Direct White-Light-Emitting Metal Organic Framework with Tunable Yellow-to-White Photoluminescence by Variation of Excitation Light. J. Am. Chem. Soc. 2009, 131, 13572–13573. [Google Scholar] [CrossRef]
- Yu, J.; Cui, Y.; Wu, C.D.; Yang, Y.; Chen, B.; Qian, G. A Two-photon Responsive Metal-Organic Framework. J. Am. Chem. Soc. 2015, 137, 4026–4029. [Google Scholar] [CrossRef]
- Deng, J.H.; Luo, J.; Mao, Y.L.; Lai, S.; Gong, Y.N.; Zhong, D.C.; Lu, T.B. π−π stacking interactions: Non-negligible forces for stabilizing porous supramolecular frameworks. Sci. Adv. 2020, 6, eaax9976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone. Molecules 2021, 26, 1695. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Li, X.; Wang, L.; Xu, Y.; Li, G. Recent Advances in Metal Organic Frameworks Based Surface Enhanced Raman Scattering Substrates: Synthesis and Applications. Molecules 2021, 26, 209. [Google Scholar] [CrossRef]
- Mylonas-Margaritis, I.; Mayans, J.; McArdle, P.; Papatriantafyllopoulou, C. ZnII and CuII-Based Coordination Polymers and Metal Organic Frameworks by the of Use of 2-Pyridyl Oximes and 1,3,5-Benzenetricarboxylic Acid. Molecules 2021, 26, 491. [Google Scholar] [CrossRef]
- Trofimova, O.Y.; Maleeva, A.V.; Ershova, I.V.; Cherkasov, A.V.; Fukin, G.K.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers. Molecules 2021, 26, 2486. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Ryan, A.A.; Aggarwal, A.; Zhu, N.; Steuber, F.W.; Senge, M.O.; Schmitt, W. 2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units. Molecules 2021, 26, 2955. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, J.Y.; Guo, R.; An, J.D.; Huo, J.Z.; Liu, Y.Y.; Shi, W.; Ding, B. Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and L-Tyrosine. Molecules 2021, 26, 3673. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, J.; Guo, H.; Dunning, S.G.; Humphrey, S.M.; Jones, R.A. Magnetism and Luminescence of a MOF with Linear Mn3 Nodes Derived from an Emissive Terthiophene-Based Imidazole Linker. Molecules 2021, 26, 4286. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Gyepes, R.; Zauškaa, L.; Bourrellyc, S. A series of four novel alkaline earth metal–organic frameworks constructed of Ca(II), Sr(II), Ba(II) ions and tetrahedral MTB linker: Structural diversity, stability study and low/high-pressure gas adsorption properties. RSC Adv. 2020, 10, 32323–32334. [Google Scholar] [CrossRef]
- Garg, A.; Almáši, M.; Paul, D.R.; Poonia, E.; Luthra, J.R.; Sharma, A. Metal-Organic Framework MOF-76 (Nd): Synthesis, Characterization, and Study of Hydrogen Storage and Humidity Sensing. Front. Energy Res. 2021, 8, 604735. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tohnai, N.; Saeki, A.; Hisaki, I. Hydrogen-bonded organic frameworks of twisted polycyclic aromatic hydrocarbon. Chem. Commun. 2020, 56, 13369–13372. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Shang, Y.; Du, J.; Yin, J.; Liu, D.; Kang, Z.; Wang, R.; Sun, D.; Jiang, J. Three Hydrogen-Bonded Organic Frameworks with Water-Induced Single-Crystal-to-Single-Crystal Transformation and High Proton Conductivity. Cryst. Growth Des. 2020, 20, 3456–3465. [Google Scholar] [CrossRef]
- Li, Y.L.; Alexandrov, E.V.; Yin, Q.; Li, L.; Fang, Z.B.; Yuan, W.; Proserpio, D.M.; Liu, T.F. Record Complexity in the Polycatenation of Three Porous Hydrogen-Bonded Organic Frameworks with Stepwise Adsorption Behaviors. J. Am. Chem. Soc. 2020, 142, 7218–7224. [Google Scholar] [CrossRef]
- Li, P.; Alduhaish, O.; Arman, H.D.; Wang, H.; Alfooty, K.; Chen, B. Solvent Dependent Structures of Hydrogen-Bonded Organic Frameworks of 2,6-Diaminopurine. Cryst. Growth Des. 2014, 14, 3634–3638. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Staples, R.J.; Zhang, J.; Shreeve, J.M. Taming nitroformate through encapsulation with nitrogen-rich hydrogen-bonded organic frameworks. Nat. Commun. 2021, 12, 2146. [Google Scholar] [CrossRef]
- Suzuki, Y.; Gutiérrez, M.; Tanaka, S.; Gomez, E.; Tohnai, N.; Yasuda, N.; Matubayasi, N.; Douhal, A.; Hisaki, I. Construction of isostructural hydrogen-bonded organic frameworks: Limitations and possibilities of pore expansion. Chem. Sci. 2021, 12, 9607–9618. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Z.; Jia, X.J.; Deng, J.H.; Zhong, J.L.; Liu, H.J.; Wang, K.J.; Zhong, D.C. A Microporous Hydrogen-Bonded Organic Framework: Exceptional Stability and Highly Selective Adsorption of Gas and Liquid. J. Am. Chem. Soc. 2013, 135, 11684–11687. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C.S. Coordination sphere hydrogen bonding as a structural element in metal–organic Frameworks. Dalton Trans. 2021, 50, 6034–6049. [Google Scholar] [CrossRef]
- Tripathy, R.J.; Aneeya, K.; Samantara, A.K.; Behera, J.N. A cobalt metal-organic framework (Co-MOF): A bi-functional electro active material for the oxygen evolution and reduction reaction. Dalton Trans. 2019, 48, 10557–10564. [Google Scholar] [CrossRef]
- Duan, J.; Mebs, S.; Laun, K.; Wittkamp, F.; Heberle, J.; Happe, T.; Hofmann, E.; Apfel, U.P.; Winkler, M.; Senger, M.; et al. Geometry of the Catalytic Active Site in [FeFe]-Hydrogenase Is Determined by Hydrogen Bonding and Proton Transfer. ACS Catal. 2019, 9, 9140–9149. [Google Scholar] [CrossRef]
- Dahl, E.W.; Szymczak, N.K. Hydrogen Bonds Dictate the Coordination Geometry of Copper: Characterization of a Square-Planar Copper(I) Complex. Angew. Chem. Int. Ed. 2016, 55, 3101–3105. [Google Scholar] [CrossRef] [Green Version]
- Hoffert, W.A.; Mock, M.T.; Appel, A.M.; Yang, J.Y. Incorporation of hydrogen-bonding functionalities into the second coordination sphere of iron-based water-oxidation catalysts. Eur. J. Inorg. Chem. 2013, 22, 3846–3857. [Google Scholar] [CrossRef] [Green Version]
- Chapovetsky, A.; Welborn, M.; Luna, J.M.; Haiges, R.; Miller , T.F., III; Marinescu, S.C. Pendant Hydrogen-Bond Donors in Cobalt Catalysts Independently Enhance CO2 Reduction. ACS Cent. Sci. 2018, 4, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, J.P.; Mullis, D.M.; Zeller, M.; Szymczak, N.K. Reductively Stable Hydrogen-Bonding Ligands Featuring Appended CF2–H Units. J. Am. Chem. Soc. 2020, 142, 8809–8817. [Google Scholar] [CrossRef]
- Santoro, A.; Bella, G.; Bruno, G.; Neri, G.; Akbari, Z.; Nicolò, F. Cocrystal versus salt, a matter of hydrogen bonds in two benzoic acid crystals. J. Mol. Struct. 2021, 1229, 129801. [Google Scholar] [CrossRef]
- Chinthal, C.H.; Kavitha, C.N.; Yathirajan, H.S.; Foro, S.; Rathore, R.S.; Glidewell, C. Fifteen 4-(2-meth-oxy-phen-yl)piperazin-1-ium salts containing organic anions: Supra-molecular assembly in zero, one, two and three dimensions. Acta Crystallogr. Sect. E Cryst. Commun. 2020, 76, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Peng, M.X. Supramolecular Architectures Constructed from Piperazine and Substituted Benzoic Acids. J. Chem. Crystallogr. 2011, 41, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, A.; Trzybiński, D. The influence of benzoate anion substituents on the crystal packing and hydrogen-bonding network of 9-aminoacridinium salts. Tetrahedron 2011, 67, 2839–2843. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisaki, I.; Miyano, T.; Tohnai, N.; Morimoto, K.; Sato, H.; Tsuzuki, S.; Miyata, M. Linkage control between molecular and supramolecular chirality in 21-helical hydrogen-bonded networks using achiral components. Nat. Commun. 2013, 4, 1787. [Google Scholar] [CrossRef] [Green Version]
- Kinbara, K.; Hashimoto, Y.; Sukegawa, M.; Nohira, H.; Saigo, K. Crystal Structures of the Salts of Chiral Primary Amines with Achiral Carboxylic Acids: Recognition of the Commonly-Occurring Supramolecular Assemblies of Hydrogen-Bond Networks and Their Role in the Formation of Conglomerates. J. Am. Chem. Soc. 1996, 118, 3441–3449. [Google Scholar] [CrossRef]
- Rani, J.; Grover, V.; Dhamija, S.; Titi, H.M.; Patra, R. Computational insight into the halogen bonded self-assembly of hexa-coordinated metalloporphyrins. Phys. Chem. Chem. Phys. 2020, 22, 11558–11566. [Google Scholar] [CrossRef]
- Sugiyama, H.; Uekusa, H. Relationship between crystal structures and photochromic properties of N-salicylideneaminopyridine derivatives. CrystEngComm 2018, 20, 2144–2151. [Google Scholar] [CrossRef]
- Kalaj, M.; Carter, K.P.; Cahill, C.L. Isolating Equatorial and Oxo Based Influences on Uranyl Vibrational Spectroscopy in a Family of Hybrid Materials Featuring Halogen Bonding Interactions with Uranyl Oxo Atoms. Eur. J. Inorg. Chem. 2017, 40, 4702–4713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.F.; Young, D.J.; Zhu, H.; Hu, F.L.; Mi, Y.; Qin, Z.; Chen, S.L.; Lang, J.P. Tuning the configuration of the flexible metal–alkene-framework affords pure cycloisomers in solid state photodimerization. Chem. Commun. 2021, 57, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, N.Y.; Lang, J.P. Single-crystal to single-crystal transformation of 1D coordination polymervia photochemical [2+2] cycloaddition reaction. Dalton Trans. 2011, 40, 2170–2172. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, U.P. Synthesis, structural, photophysical and thermal properties of some praseodymium(III) complexes. J. Coord. Chem. 2008, 61, 2663–2674. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Tang, Y.H.; Kang, Y.; Li, Z.J.; Hu, R.F.; Cheng, J.K.; Wen, Y.H.; Yao, Y. Crystal structures and 31P NMR spectra of two trinuclear molybdenum clusters coordinated by p-chlorobenzoate: Mo3S4(DTP)3(p-ClC6H4COO)(Py)·EtOH and Mo3S4(DTP)3(p-ClC6H4COO)(DMF). J. Mol. Struct. 2004, 707, 235–239. [Google Scholar] [CrossRef]
- Wang, N.; Li, B. Synthesis, Crystal Structure and Cytotoxic Property of Bis(4-chlorobenzoato)bis[1-(2-aminoethyl)piperidine]disilver(I). J. Chem. Crystallogr. 2011, 41, 219–222. [Google Scholar] [CrossRef]
- Carter, K.P.; Zulatoa, C.H.F.; Cahill, C.L. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm 2014, 16, 10189–10202. [Google Scholar] [CrossRef] [Green Version]
- Deka, K.; Sarma, R.J.; Baruah, J.B. Nitrogen-oxygen bond formation during oxidative reactions of copper(II)benzoate complexes having 3,5-dimethylpyrazole. Inorg. Chem. Commun. 2006, 9, 931–934. [Google Scholar] [CrossRef]
- Sun, D.; Liu, F.J.; Hao, H.J.; Li, Y.H.; Huang, R.B.; Zheng, L.S. Six low-dimensional silver(I) coordination complexes derived from 2-aminobenzonitrile and carboxylates. Inorg. Chimi. Acta 2012, 387, 271–276. [Google Scholar] [CrossRef]
- Halaška, J.; Lokaj, J.; Jomová, K.; Růžičková, Z.; Mazúr, M.; Moncol, J. Formation of supramolecular hydrogen-bonding chains and networks from copper (II) halogenobenzoates with N-methylnicotinamide: Supramolecular isomerism. Polyhedron 2020, 175, 114237. [Google Scholar] [CrossRef]
- Xu, T.Y.; Nie, H.J.; Li, J.M.; Shi, Z.F. Highly selective sensing of Fe3+/Hg2+ and proton conduction using two fluorescent Zn(II) coordination polymers. Dalton Trans. 2020, 49, 11129–11141. [Google Scholar] [CrossRef]
- Xu, T.Y.; Nie, H.J.; Li, J.M.; Shi, Z.F. Luminescent Zn(II)/Cd(II) coordination polymers based on 1-(tetrazol-5-H)-3,5-bis(1-triazole)benzene for sensing Fe3+, Cr2O72−, and CrO42− in water. J. Solid State Chem. 2020, 287, 121342. [Google Scholar] [CrossRef]
- Xu, T.Y.; Li, J.M.; Han, Y.H.; Wang, A.R.; He, K.H.; Shi, Z.F. A new 3D four-fold interpenetrated dia-like luminescent Zn(II)-based metal–organic framework: The sensitive detection of Fe3+, Cr2O72−, and CrO42− in water, and nitrobenzene in ethanol. New J. Chem. 2020, 44, 4011–4022. [Google Scholar] [CrossRef]
- Xu, T.Y.; Zhao, Y.L.; Li, J.M.; Shi, Z.F. Co(II) Coordination Polymer Based on Bis(carboxyl) Dibenzylamine and Bis(imidazole) Biphenyl Ligands: Synthesis, Crystal Structure and Detection for Fe3+ Ions in Water. Chinese J. Inorg. Chem. 2020, 36, 1735–1743. [Google Scholar]
- Xu, T.Y.; Wang, H.; Li, J.M.; Zhao, Y.L.; Han, Y.H.; Wang, X.L.; He, K.H.; Wang, A.R.; Shi, Z.F. A water-stable luminescent Zn(II) coordination polymer based on 5-sulfosalicylic acid and 1,4-bis(1H-imidazol-1-yl)benzene for highly sensitive and selective sensing of Fe3+ ion. Inorg. Chim. Acta 2019, 493, 72–80. [Google Scholar] [CrossRef]
- Li, J.M.; Xu, T.Y.; Zhao, Y.L.; Hu, X.L.; He, K.H. Two 6/10-connected Cu12S6 cluster-based organic frameworks: Crystal structure and proton conduction. Dalton Trans. 2021, 50, 7484–7495. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Li, J.L. Synthesis, Crystal and Hirshfeld Surface Structure of a 1D Ni(II) Coordination Polymer: Luminescent Detection for Fe3+ in H2O. ChemistrySelect 2021, 6, 4135–4142. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Liu, M.; Yin, C.; Chen, P.; Zhang, M.; Parkin, S.; Zhou, P.; Li, T.; Yu, F.; Long, S. sp2 C-H···Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid. CrystEngComm 2017, 19, 4345–4354. [Google Scholar] [CrossRef]
- Aakerö, C.B.; Evans, T.A.; Seddon, K.R.; Pálinkó, I. The C-H···Cl hydrogen bond: Does it exist? New J. Chem. 1999, 23, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudkhani, A.H.; Langerb, V.; Casar, B.M. Two-dimensional network of C-H···Cl-Co hydrogen bonds in the structure of 1,1,4,4-tetraisopropyl-piperazinium tetrachlorocobaltate(II). Acta Cryst. 2001, E57, m393–m395. [Google Scholar]
- Jiang, G.; Bai, J.; Xing, H.; Li, Y.; You, X. A Tetrahedral Water Tetramer in a Zeolite-like Metal-Organic Framework Constructed from {(H3O)2[Fe6O{(OCH2)3CCH3}4Cl6].4H2O}. Cryst. Growth Des. 2006, 6, 1264–1266. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Hay, B.P. Are C-H Groups Significant Hydrogen Bonding Sites in Anion Receptors? Benzene Complexes with Cl−, NO3−, and ClO4−. J. Am. Chem. Soc. 2005, 127, 8282–8283. [Google Scholar] [CrossRef] [PubMed]
- Tresca, B.W.; Zakharov, L.N.; Carroll, C.N.; Johnson, D.W.; Haley, M.M. Aryl C-H···Cl− hydrogen bonding in a fluorescent anion sensor. Chem. Commun. 2013, 49, 7240–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.; Gou, Q.; Evangelisti, L.; Vallejo-López, M.; Lesarri, A.; Cocineroc, E.J.; Caminati, W. Competition between weak hydrogen bonds: C-H···Cl is preferred to C-H···F in CH2ClF-H2CO, as revealed by rotational spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12261–12265. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Feng, D.-D.; Tang, J.; Zhao, Y.-D.; Li, J.; Yang, J.; Kim, C.K.; Su, F. A Water-Stable Zinc(II)-Organic Framework as a Multi-responsive Luminescent Sensor for Toxic Heavy Metal Cations, Oxyanions and Organochlorine Pesticides in Aqueous Solution. Dalton Trans. 2019, 48, 16776–16785. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, T.; Pan, D.; Zhang, X.; Wang, Y.; Shi, Y.-C.; Yu, H. Synthesis, structure, and photoluminescence properties of coordination polymers of 4,4′,4″,4‴-tetrakiscarboxyphenylsilane and 3,5-bis(1′,2′,4′-triazol-1′-yl)pyridine. CrystEngComm 2020, 22, 534–545. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Q.-Y.; Zhao, Y.-L.; Li, J.-M.; Ouyang, M. Construction of Two Stable Co(II)-Based Hydrogen-Bonded Organic Frameworks as a Luminescent Probe for Recognition of Fe3+ and Cr2O72− in H2O. Molecules 2021, 26, 5955. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195955
Weng Q-Y, Zhao Y-L, Li J-M, Ouyang M. Construction of Two Stable Co(II)-Based Hydrogen-Bonded Organic Frameworks as a Luminescent Probe for Recognition of Fe3+ and Cr2O72− in H2O. Molecules. 2021; 26(19):5955. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195955
Chicago/Turabian StyleWeng, Qi-Ying, Ya-Li Zhao, Jia-Ming Li, and Miao Ouyang. 2021. "Construction of Two Stable Co(II)-Based Hydrogen-Bonded Organic Frameworks as a Luminescent Probe for Recognition of Fe3+ and Cr2O72− in H2O" Molecules 26, no. 19: 5955. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195955