Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Structure Phenomena in Fatty Acids
2.2. Infrared Spectra of Saturated Monoacids and Their Metal Salts
2.2.1. Monoacids
The Acidic Hydroxyl Stretching Region
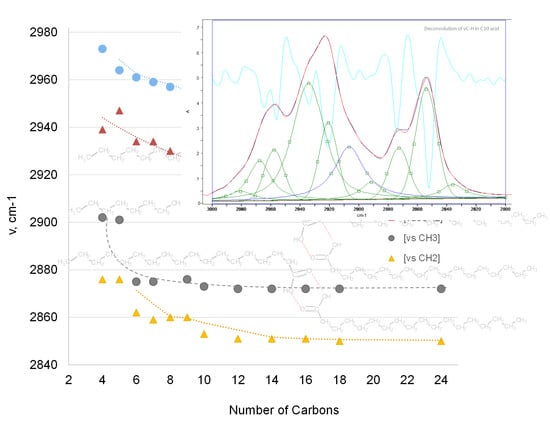
The C‒H Stretching Region
The Acidic Carbonyl Stretching Region
The CH2 and CH3 Bending Region
The CH2 Twisting and Wagging Region
Hydroxyl C‒O Stretch and C‒O‒H Bending Region
The CH2 Rocking Region
Infrared Maxima and Crystallization
2.2.2. Fatty Monoacid Metal Salts
The Carboxylate Stretching
The C‒H Vibrations
2.3. Infrared Spectra of Selected Diacids and Their Metal Salts
2.3.1. Azelaic and Suberic Acids
2.3.2. Diacid Metal Salts
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fatty Acid Metal Salts
3.3. Fourier Transform Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Symbols
References
- Mills, J.S. The Organic Chemistry of Museum Objects, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Mills, J.S. The Gas Chromatographic Examination of Paint Media. Part, I. Fatty Acid Composition and Identification of Dried Oil Films. Stud. Conserv. 1966, 11, 92–107. [Google Scholar] [CrossRef]
- Mills, J.S.; White, R. The gas-chromatographic examination of paint media. Part II. Some examples of medium identification in paintings by fatty acid analysis. Stud. Conserv. 1972, 17, 721–728. [Google Scholar] [CrossRef]
- Nawar, W.W. Thermal degradation of lipids. J. Agric. Food Chem. 1969, 17, 18–21. [Google Scholar] [CrossRef]
- Casadio, F.; Keune, K.; Noble, P.; Van Loon, A.; Hendriks, E.; Centeno, S.A.; Osmond, G. Metal Soaps in Art; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Hermans, J.J. Metal Soaps in Oil Paint: Structure, Mechanisms and Dynamics; University of Amsterdam: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Keune, K.; Boon, J.J. Analytical Imaging Studies of Cross-Sections of Paintings Affected by Lead Soap Aggregate Formation. Stud. Conserv. 2007, 52, 161–176. [Google Scholar] [CrossRef]
- Vichi, A.; Eliazyan, G.; Kazarian, S.G. Study of the Degradation and Conservation of Historical Leather Book Covers with Macro Attenuated Total Reflection-Fourier Transform Infrared Spectroscopic Imaging. ACS Omega 2018, 3, 7150–7157. [Google Scholar] [CrossRef]
- Koupadi, K.; Boyatzis, S.C.; Roumpou, M. Organic remains in archaeological objects: Investigating their surviving profile in early Christian Egyptian vessels with Fou-rier Transform Infrared Spectroscopy and Gas Chromatog-raphy-Mass Spectrometry. Heritage 2021. (submitted). [Google Scholar]
- Regert, M.; Bland, H.A.; Dudd, S.N.; Bergen, P.V.; Evershed, R.P. Free and bound fatty acid oxidation products in archaeological ceramic vessels. Proc. R. Soc. B Biol. Sci. 1998, 265, 2027–2032. [Google Scholar] [CrossRef]
- McGovern, P.E.; Hall, G.R. Charting a Future Course for Organic Residue Analysis in Archaeology. J. Archaeol. Method Theory 2016, 23, 592–622. [Google Scholar] [CrossRef]
- Historic England. Organic Residue Analysis and Archaeology; Supporting Information; Historic England: London, UK, 2017; Volume 32. [Google Scholar]
- Orna, M.V.; Lambert, J.B. New directions in archaeological chemistry. In Archaeological Chemistry; American Chemical Society: Washingtom, DC, USA, 1996; pp. 1–9. [Google Scholar]
- Price, T.D.; Burton, J.H. Archaeological Chemistry. In An Introduction to Archaeological Chemistry; Springer: New York, NY, USA, 2011; pp. 1–24. [Google Scholar]
- Lambert, J.B. Traces of the Past: Unraveling the Secrets of Archaeology Through Chemistry, 1st ed.; Perseus Books: Boston, MA, USA, 1998. [Google Scholar]
- Evershed, R.P.; Payne, S.; Sherratt, A.G.; Copley, M.S.; Coolidge, J.; Urem-Kotsu, D.; Burton, M.M. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 2008, 455, 528–531. [Google Scholar] [CrossRef]
- Maniatis, Y.; Tsirtsoni, Z. Characterization of a black residue in a decorated Neolithic pot from Dikili Tash, Greece: An unexpected result. Archaeometry 2002, 44, 229–239. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Jacomet, S.; Schibler, J. Chemical analyses of organic residues in archaeological pottery from Arbon Bleiche 3, Switzerland—Evidence for dairying in the late Neolithic. J. Archaeol. Sci. 2006, 33, 1–13. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milanob, A.; Russob, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Mitkidou, S.; Dimitrakoudi, E.; Urem-Kotsou, D.; Papadopoulou, D.; Kotsakis, K.; Stratis, J.A.; Stephanidou-Stephanatou, I. Organic residue analysis of Neolithic pottery from North Greece. Microchim. Acta 2008, 160, 493–498. [Google Scholar] [CrossRef]
- Raven, A.M.; van Bergen, P.F.; Stott, A.W.; Dudd, S.N.; Evershed, R.P. Formation of long-chain ketones in archaeological pottery vessels by pyrolysis of acyl lipids. J. Anal. Appl. Pyrolysis 1997, 40–41, 267–285. [Google Scholar] [CrossRef]
- Suryanarayan, A.; Cubas, M.; Craig, O.E.; Herone, C.P.; Shinde, V.S.; Singh, R.N.; O’Connell, T.C.; Petrie, C.A. Lipid residues in pottery from the Indus Civilisation in northwest India. J. Archaeol. Sci. 2021, 125, 105291. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, M. Infrared spectroscopic characterization of residues on archaeological pottery through different spectra acquisition modes. Vib. Spectrosc. 2015, 76, 48–54. [Google Scholar] [CrossRef]
- Shillito, L.M.; Almond, M.J.; Wicks, K.; Marshall, L.-J.R.; Matthews, W. The use of FT-IR as a screening technique for organic residue analysis of archaeological samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 120–125. [Google Scholar] [CrossRef]
- McGovern, P.E.; Glusker, D.L.; Exner, L.J.; Voigt, M.M. Neolithic resinated wine. Nature 1996, 381, 480–481. [Google Scholar] [CrossRef]
- Stern, B.; Heron, C.; Tellefsen, T.; Serpico, M. New investigations into the Uluburun resin cargo. J. Archaeol. Sci. 2008, 35, 2188–2203. [Google Scholar] [CrossRef]
- Hammann, S.; Scurr, D.J.; Alexander, M.R.; Cramp, L.J.E. Mechanisms of lipid preservation in archaeological clay ceramics revealed by mass spectrometry imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 14688–14693. [Google Scholar] [CrossRef]
- Regert, M. Analytical strategies for discriminating archeological fatty substances from animal origin. Mass Spectrom. Rev. 2011, 30, 177–220. [Google Scholar] [CrossRef] [PubMed]
- Mottram, H.R.; Evershed, R.P. Structure Analysis of Triacylglycerol Positional Isomers Using Atmospheric Pressure Chemical Ionisation Mass Spectrometry. Tetrahedron Lett. 1996, 37, 8593–8596. [Google Scholar] [CrossRef]
- Mottram, H.; Dudd, S.; Lawrence, G.; Stott, A.W.; Evershed, R.P. New chromatographic, mass spectrometric and stable isotope approaches to the classification of degraded animal fats preserved in archaeological pottery. J. Chromatogr. A 1999, 833, 209–221. [Google Scholar] [CrossRef]
- Heron, C.; Evershed, R.P. The Analysis of Organic Residues and the Study of Pottery Use The Analysis of Organic Residues and the Study of Pottery Use. Archaeol. Method Theory 1993, 5, 247–284. [Google Scholar]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [Green Version]
- Izzo, F.C.; Zendri, E.; Bernardi, A.; Balliana, E.; Sgobbi, M. The study of pitch via gas chromatography–mass spectrometry and Fourier-transformed infrared spectroscopy: The case of the Roman amphoras from Monte Poro, Calabria (Italy). J. Archaeol. Sci. 2013, 40, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Regert, M.; Garnier, N.; Decavallas, O.; Cren-Olivé, C.; Rolando, C. Structural characterization of lipid constituents from natural substances preserved in archaeological environments. Meas. Sci. Technol. 2003, 14, 1620–1630. [Google Scholar] [CrossRef]
- Craig, O.E.; Steele, V.J.; Fischer, A.; Hartz, S.; Andersen, S.H.; Donohoe, P.; Glykou, A.; Saul, H.; Jones, D.M.; Koch, E.; et al. Ancient lipids reveal continuity in culinary practices across the transition to agriculture in Northern Europe. Proc. Natl. Acad. Sci. USA 2011, 108, 17910–17915. [Google Scholar] [CrossRef] [Green Version]
- Roffet-Salque, M.; Dunne, J.; Altoft, D.T.; Casanova, E.; Cramp, L.J.E.; Smyth, J.; Whelton, H.L.; Evershed, R.P. From the inside out: Upscaling organic residue analyses of archaeological ceramics. J. Archaeol. Sci. Rep. 2017, 16, 627–640. [Google Scholar] [CrossRef] [Green Version]
- Gregg, M.W.; Slater, G.F. A new method for extraction, isolation and transesterification of free fatty acids from archaeological pottery. Archaeometry 2010, 52, 833–854. [Google Scholar] [CrossRef]
- Šoberl, L.; Žibrat Gašparič, A.; Budja, M.; Evershed, R.P. Early herding practices revealed through organic residue analysis of pottery from the early Neolithic rock shelter of Mala Triglavca, Slovenia. Doc. Praehist. 2008, 35, 253–260. [Google Scholar] [CrossRef] [Green Version]
- McGovern, P.E.; Glusker, D.L.; Moreau, R.A.; Nuñez, A.; Beck, C.W.; Simpson, E.; Butrym, E.D.; Exner, L.J.; Stout, E.C. A funerary feast fit for King Midas. Nature 1999, 402, 863–864. [Google Scholar] [CrossRef]
- Boyatzis, S.C.; Kotzamani, D.; Phoca, A.; Karydi, G.M.Z.A.V.K. Characterization of Organic Remains Found in Copper Alloy Vessels of the Benaki Museum Collection with Fourier Transform Mid-Infrared Spectroscopy. In Proceedings of the 3rd ARCH_RNT, Kalamata, Greece, 3–5 October 2012; Zacharias, N., Ed.; University of the Peloponnese: Tripoli, Greece, 2014. [Google Scholar]
- Evershed, R.P.; Dudd, S.N.; Copley, M.S.; Berstan, B.; Stott, A.W.; Mottram, H.; Buckley, S.A.; Crossman, Z. Chemistry of archaeological animal fats. Acc. Chem. Res. 2002, 35, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.S.; Macdonald, F.V.; Braun, K.L. Lipid Residue Analysis of Archaeological Pottery: An Introductory Laboratory Experiment in Archaeological Chemistry. J. Chem. Educ. 2017, 94, 1309–1313. [Google Scholar] [CrossRef]
- Heron, C.; Stacey, R. Archaeology: Uses of Chromatography in; Academic Press: Cambridge, MA, USA, 2000; pp. 2083–2089. [Google Scholar]
- Stern, B.; Heron, C.; Serpico, M.; Bourriau, J. A comparison of methods for establishing fatty acid concentration gradients across potsherds: A case study using Late Bronze Age Canaanite amphorae. Archaeometry 2000, 42, 399–414. [Google Scholar] [CrossRef]
- Pitonzo, R.; Armetta, F.; Saladino, M.L.; Oliveri, F.; Tusa, S.; Caponetti, E. Application of Gas Chromatography coupled with Mass Spectroscopy (GC/MS) to the analysis of archaeological ceramic amphorae belonging to the Carthaginian fleet that was defeated in the Egadi battle (241 B.C.). Acta IMEKO 2017, 6, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Sciutto, G.; Oliveri, P.; Prati, S.; Quaranta, M.; Lanteri, S.; Mazzeo, R. Analysis of paint cross-sections: A combined multivariate approach for the interpretation of μATR-FTIR hyperspectral data arrays. Anal. Bioanal. Chem. 2013, 405, 625–633. [Google Scholar] [CrossRef]
- Mazzeo, R.; Prati, S.; Quaranta, M.; Joseph, E.; Kendix, E.; Galeotti, M. Attenuated total reflection micro FTIR characterisation of pigment-binder interaction in reconstructed paint films. Anal. Bioanal. Chem. 2008, 392, 65–76. [Google Scholar] [CrossRef]
- Spring, M.; Ricci, C.; Peggie, D.A.; Kazarian, S.G. ATR-FTIR imaging for the analysis of organic materials in paint cross sections: Case studies on paint samples from the National Gallery, London. Anal. Bioanal. Chem. 2008, 392, 37–45. [Google Scholar] [CrossRef]
- Bonaduce, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical approaches based on gas chromatography mass spectrometry (GC/MS) to study organic materials in artworks and archaeological objects. Top Curr. Chem. 2016, 374, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Griffiths, P.R.; Haseth, J.A. Fourier Transform Infrared Spectrometry, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Griffiths, P.R. Introduction to Vibrational Spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Stuart, B.H. Analytical Techniques in Materials Conservation; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Meilunas, R.J.; Bentsen, J.G.; Steinberg, A. Analysis of aged paint binders by ftir spectroscopy. Stud. Conserv. 1990, 35, 33–51. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 10815–10837. [Google Scholar]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Springer: Dordrecht, The Netherlands, 1975. [Google Scholar]
- Shurvell, H.F. Spectra—Structure Correlations in the Mid- and Far-Infrared. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Mayo, D.; Miller, F.; Hannah, R. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Szymanski, H.A. Interpreted Infrared Spectra_ Volume 1-Springer US (1964); Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Silverwood, I.P.; Keyworth, C.W.; Brown, N.J.; Shaffer, M.S.P.; Williams, C.K.; Hellgardt, K.; Kelsall, G.H.; Kazarian, S.G. An attenuated total reflection fourier transform infrared (ATR FT-IR) spectroscopic study of gas adsorption on colloidal stearate-capped ZnO catalyst substrate. Appl. Spectrosc. 2014, 68, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Andrew Chan, K.L.; Kazarian, S.G. Attenuated total reflection Fourier-transform infrared (ATR-FTIR) imaging of tissues and live cells. Chem. Soc. Rev. 2016, 45, 1850–1864. [Google Scholar] [CrossRef]
- Umemura, J. Reflection-Absorption Spectroscopy of Thin Films on Metallic Substrates. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Claybourn, M. External Reflection Spectroscopy. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Bitossi, G.; Giorgi, R.; Mauro, M.; Salvadori, B.; Dei, L. Spectroscopic Techniques in Cultural Heritage Conservation: A Survey. Appl. Spectrosc. Rev. 2005, 40, 187–228. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Bonacini, I.; Mazzeo, R. New Frontiers in Application of FTIR Microscopy for Characterization of Cultural Heritage Materials. Top. Curr. Chem. 2016, 374, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.; Joseph, E.; Prati, S.; Millemaggi, A. Attenuated Total Reflection-Fourier transform infrared microspectroscopic mapping for the characterisation of paint cross-sections. Anal. Chim. Acta 2007, 599, 107–117. [Google Scholar] [CrossRef]
- Van der Weerd, J.; van Loon, A.; Boon, J.J. FTIR Studies of the Effects of Pigments on the Aging of Oil. Stud. Conserv. 2005, 50, 3–22. [Google Scholar] [CrossRef]
- Osmond, G.; Boon, J.J.; Puskar, L.; Drennan, J. Metal Stearate Distributions in Modern Artists’ Oil Paints: Surface and Cross-Sectional Investigation of Reference Paint Films Using Conventional and Synchrotron Infrared Microspectroscopy. Appl. Spectrosc. 2012, 66, 1136–1144. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; van Loon, A.; Stols-Witlox, M.J.N.; Corkery, R.W.; Iedema, P.D. The synthesis of new types of lead and zinc soaps: A source of information for the study of oil paint degradation. In Proceedings of the ICOM-CC 17th Triennial Conference, Melbourne, Australia, 15–19 September 2014; p. 1603. [Google Scholar]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Corkery, R.W.; Iedema, P.D. Ionomer-like structure in mature oil paint binding media. RSC Adv. 2016, 6, 93363–93369. [Google Scholar] [CrossRef] [Green Version]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. An infrared spectroscopic study of the nature of zinc carboxylates in oil paintings. J. Anal. At. Spectrom. 2015, 30, 1600–1608. [Google Scholar] [CrossRef] [Green Version]
- Casadio, F.; Bellot-Gurlet, L.; Paris, C. Factors Affecting the Reactivity of Zinc Oxide with Different Drying Oils: A Vibrational Spectroscopy Study. In Metal Soaps in Art; Springer Publishing: Cham, Switzerland, 2019; pp. 153–170. [Google Scholar]
- Banti, D.; La Nasa, J.; Tenorio, A.L.; Modugno, F.; Berg, K.J.V.A.; Lee, J.; Ormsby, B.; Burnstock, A.; Bonaduce, I. A molecular study of modern oil paintings: Investigating the role of dicarboxylic acids in the water sensitivity of modern oil paints. RSC Adv. 2018, 8, 6001–6012. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Beltran, V.; Ramer, G.; Georges, P.; Dilworthy, P.; Tyler, M.; Andrea, C.; Barbara, B. Revealing the Distribution of Metal Carboxylates in Oil Paint from the Micro- to Nanoscale. Angew. Chem. Int. Ed. 2019, 58, 11652–11656. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. The crystallization of metal soaps and fatty acids in oil paint model systems. Phys. Chem. Chem. Phys. 2016, 18, 10896–10905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinet, L.; Corbeil, M.C. The characterization of metal soaps. Stud. Conserv. 2003, 48, 23–40. [Google Scholar] [CrossRef]
- Oudemans, T.F.M.; Boon, J.J.; Botto, R.E. FTIR and solid-state 13C CP/MAS NMR spectroscopy of charred and non-charred solid organic residues preserved in roman iron age vessels from the Netherlands. Archaeometry 2007, 49, 571-294. [Google Scholar] [CrossRef]
- McGovern, P.E. Uncorking the Past. The Quest for Wine, Beer, and Other Alcoholic Beverages; University of California Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Thickett, D.; Pretzel, B. Micro-spectroscopy: A powerful tool to understand deterioration. e-Preserv. Sci. 2010, 7, 158–164. [Google Scholar]
- Plater, M.J.; De Silva, B.; Gelbrich, T.; Hursthouse, M.; Higgitt, C.; Saunders, D. The characterisation of lead fatty acid soaps in “protrusions” in aged traditional oil paint. Polyhedron 2003, 22, 3171–3179. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; van Loon, A.; Corkery, R.W.; Iedema, P.D. The molecular structure of three types of long-chain zinc(II) alkanoates for the study of oil paint degradation. Polyhedron 2014, 81, 335–340. [Google Scholar] [CrossRef]
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.A.; Melo, M.J. Supporting Information: Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J. Raman. Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Gönen, M.; Öztürk, S.; Balköse, D.; Okur, S.; Ülkü, S. Preparation and characterization of calcium stearate powders and films prepared by precipitation and Langmuir-Blodgett techniques. Ind. Eng. Chem. Res. 2010, 49, 1732–1736. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L.; Brockway, L.O. The Structure of the Carboxyl Group: I. The Investigation of Formic Acid by the Diffraction of Electrons. Proc. Natl. Acad. Sci. USA 1934, 20, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Kishida, S.; Nakamoto, K. Normal coordinate analyses of hydrogen-bonded compounds. II. Dimeric formic acid and acetic acid. J. Chem. Phys. 1964, 41, 1558–1563. [Google Scholar] [CrossRef]
- Witkowski, A. Infrared Spectra of the Hydrogen-Bonded Carboxylic Acids. J. Chem. Phys. 1967, 47, 3645–3648. [Google Scholar] [CrossRef]
- Kaneko, F.; Tashiro, K.; Kobayashi, M. Polymorphic transformations during crystallization processes of fatty acids studied with FT-IR spectroscopy. J. Cryst. Growth 1999, 198–199, 1352–1359. [Google Scholar] [CrossRef]
- Kaneko, F.; Ishikawa, E.; Kobayashi, M.; Suzuki, M. Structural study on polymorphism of long-chain dicarboxylic acids using oblique transmission method for micro FT-IR spectrometers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 9–18. [Google Scholar] [CrossRef]
- Thalladi, V.R.; Nüsse, M.; Boese, R. The melting point alternation in α,ω-alkanedicarboxylic acids. J. Am. Chem. Soc. 2000, 122, 9227–9236. [Google Scholar] [CrossRef]
- Badger, R.M.; Bauer, S.H. Spectroscopic studies of the hydrogen bond I. A photometric investigation of the association equilibrium in the vapor of acetic acid. J. Chem. Phys. 1937, 5, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Badger, R.M.; Bauer, S.H. Spectroscopic studies of the hydrogen bond. II. The shift of the O-H vibrational frequency in the formation of the hydrogen bond. J. Chem. Phys. 1937, 5, 839–851. [Google Scholar] [CrossRef]
- Fox, J.J.; Martin, A.E. Investigations of infra-red spectra-absorption of some hydroxy compounds in the region of 3 μ. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 162, 419–441. [Google Scholar] [CrossRef] [Green Version]
- Buswell, A.M.; Rodebush, W.H.; Roy, M.F. Infrared Absorption Studies. V. Association in the Carboxylic Acids. J. Am. Chem. Soc. 1938, 60, 2239–2244. [Google Scholar] [CrossRef]
- Davies, M.M.; Sutherland, G.B.B.M. Hydroxyl Frequency in Carboxylic Acids. Nature 1938, 141, 372–373. [Google Scholar] [CrossRef]
- Cannon, C.G. The nature of hydrogen bonding. Spectrochim. Acta 1958, 10, 341–368. [Google Scholar] [CrossRef]
- Hadži, D. Hydrogen Bonding. In Proceedings of the Symposium on Hydrogen Bonding, Ljubljana, Slovenia, 29 July–3 August 1957; Pergamon Press: Oxford, UK, 1959. [Google Scholar]
- Nakamoto, K.; Margoshes, M.; Rundle, R. E Stretching Frequencies as a Function of Distances in Hydrogen Bonds. J. Am. Chem. Soc. 1955, 77, 6480–6486. [Google Scholar] [CrossRef]
- Hadzi, D.; Sheppard, N. The infra-red absorption bands associated with the COOH and COOD groups in dimeric carboxylic acids. I. The region from 1500 to 500 cm−1. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1953, 216, 247–266. [Google Scholar] [CrossRef]
- Bratož, S.; Hadži, D.; Sheppard, N. The infra-red absorption bands associated with the COOH and COOD groups in dimeric carboxylic acid-II. The region from 3700 to 1500 cm−1. Spectrochim. Acta 1956, 8, 249–261. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen Bonding—New Insights, 1st ed.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Bezrodna, T. Temperature dynamics of dimer formation in behenic acid: FT-IR spectroscopic study. J. Mol. Struct. 2013, 1040, 112–116. [Google Scholar] [CrossRef]
- Li, Y.-M.; Sun, S.-Q.; Zhou, Q.; Qin, Z.; Tao, J.-X.; Wang, J.; Fang, X. Identification of American ginseng from different regions using FT-IR and two-dimensional correlation IR spectroscopy. Vib. Spectrosc. 2004, 36, 227–232. [Google Scholar] [CrossRef]
- Silva, L.F.; Andrade-Filho, T.; Freire, P.T.C. Polarized Raman and Infrared Spectroscopy and ab Initio Calculation of Palmitic and Stearic Acids in the Bm and C Forms. J. Phys. Chem. A 2004, 121, 4830–4842. [Google Scholar] [CrossRef]
- Meiklejohn, R.A.; Meyer, R.J.; Aronovic, S.M.; Schuette, H.A.; Meloche, V.W. Characterization of Long-Chain Fatty Acids by Infrared Spectroscopy. Anal. Chem. 1957, 29, 329–334. [Google Scholar] [CrossRef]
- De Ruig, W.G. Infrared Spectra of Monoacid Triglycerides with Some Applications to Fat Analysis; Center for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1971. [Google Scholar]
- Corish, P.J.; Chapman, D. The infrared spectra of some monocarboxylic acids. J. Chem. Soc. 1957, 18, 1746. [Google Scholar] [CrossRef]
- Chapman, D. Infrared spectroscopy of lipids. J. Am. Oil Chem. Soc. 1965, 42, 353–371. [Google Scholar] [CrossRef]
- Sinclair, R.G.; McKay, A.F.; Jones, R.N. The Infrared Absorption Spectra of Saturated Fatty Acids and Esters. J. Am. Chem. Soc. 1952, 74, 2570–2575. [Google Scholar] [CrossRef]
- Comí, M.; Fernández, M.; Santamaría, A.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cadiz, V. Carboxylic Acid Ionic Modification of Castor-Oil-Based Polyurethanes Bearing Amine Groups: Chemically Tunable Physical Properties and Recyclability. Macromol. Chem. Phys. 2017, 218, 1700379. [Google Scholar] [CrossRef]
- Cheng, Q.; Cao, Y.; Yang, L.; Zhang, P.-P.; Wang, K.; Wang, H.-J. Synthesis of titania microspheres with hierarchical structures and high photocatalytic activity by using nonanoic acid as the structure-directing agent. Mater. Lett. 2011, 65, 2833–2835. [Google Scholar] [CrossRef]
- Ali, H.; Ghareeb, M.M.; Al-remawi, M.; Al-akayleh, F.T. New insight into single phase formation of capric acid/menthol eutectic mixtures by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Trop. J. Pharm. Res. 2020, 19, 361–369. [Google Scholar] [CrossRef]
- Roy, R.S. Spectroscopic studies of long chain fatty acids. Pyridine—Fatty acid—Carbon tetrachloride system. Spectrochim. Acta 1966, 22, 1877–1887. [Google Scholar] [CrossRef]
- Suzuki, M.; Shimanouchi, T. Infrared and Raman spectra of adipic acid crystal. J. Mol. Spectrosci. 1969, 29, 415–425. [Google Scholar] [CrossRef]
- Mikawa, Y.; Brasch, J.W.; Jakobsen, R.J. Polarized ir spectra of single crystals of propanoic acid. J. Mol. Struct. 1968, 3, 103–117. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 87th ed.; CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zerbi, G.; Conti, G.; Minoni, G.; Pison, S.; Bigotto, A. Premelting phenomena in fatty acids: An infrared and Raman study. J. Phys. Chem. 1987, 91, 2386–2393. [Google Scholar] [CrossRef]
- Conti, G.; Minoni, G.; Zerbi, G. E → C phase transition in fatty acids: A spectroscopic study. J. Mol. Struct. 1984, 118, 237–243. [Google Scholar] [CrossRef]
- Flett, M.S.C. The characteristic infra-red frequencies of the carboxylic acid group. J. Chem. Soc. 1951, 41, 962. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, D.; Wang, Y.; Zhao, G. Molecular structure and hydrogen bonds in solid dimethylol propionic acid (DMPA). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2713–2722. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, Z.; Zhang, G.; Guo, X.; Ma, M. Comparative study of the H-bond and FTIR spectra between 2,2-hydroxymethyl propionic acid and 2,2-hydroxymethyl butanoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 449–453. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Li, H.W.; Strauss, H.L.; Snyder, R.G. Differences in the IR methylene rocking bands between the crystalline fatty acids and n-alkanes: Frequencies, intensities, and correlation splitting. J. Phys. Chem. A 2004, 108, 6629–6642. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Vibrational Spectroscopy of Lipids. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Jones, R.N. The Effects of Chain Length on the Infrared Spectra of Fatty Acids and Methyl Esters. Can. J. Chem. 1962, 40, 321–333. [Google Scholar] [CrossRef]
- Lambert, J.B.; Shurvell, H.F.; Cooks, R.G. Introduction to Organic Spectroscopy, 1st ed.; Macmillan: New York, NY, USA, 1987. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Zbinden, R. Infrared Spectroscopy of High Polymers; Academic Press: New York, NY, USA; London, UK, 1964. [Google Scholar]
- Snyder, R.G.; Schachtschneider, J.H. Vibrational analysis of the n-paraffins—I. Assignments of infrared bandsin the spectra of C3H8 through n-C19H40. Spectrochim. Acta 1963, 19, 85–116. [Google Scholar] [CrossRef]
- Jones, R.N.; McKay, A.F.; Sinclair, R.G. Band Progressions in the Infrared Spectra of Fatty Acids and Related Compounds. J. Am. Chem. Soc. 1952, 74, 2575–2578. [Google Scholar] [CrossRef]
- Boyatzis, S.; Douvas, A.M.; Argyropoulos, V.; Siatou, A.; Vlachopoulou, M. Characterization of a water-dispersible metal protective coating with fourier transform infrared spectroscopy, modulated differential scanning calorimetry, and ellipsometry. Appl. Spectrosc. 2012, 66, 580–590. [Google Scholar] [CrossRef]
- Kirby, E.M.; Evans-Vader, M.J.; Brown, M.A. Determination of the length of polymethylene chains in salts of saturated and unsaturated fatty acids by infrared spectroscopy. J. Am. Oil Chem. Soc. 1965, 42, 437–446. [Google Scholar] [CrossRef]
- Chapman, D. The 720 cm–1 band in the infrared spectra of crystalline long-chain compounds. J. Chem. Soc. 1957, 4489–4491. [Google Scholar] [CrossRef]
- Ross, R.A.; Takacs, A. Heterogeneous Reactions of Aluminum and Copper Surfaces with Stearic Acid. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 280–286. [Google Scholar] [CrossRef]
- Ross, R.A.; Takacs, A.M. Surface reactions of ethyl stearate and stearic acid with zinc, manganese and their oxides. Surf. Technol. 1984, 21, 361–377. [Google Scholar] [CrossRef]
- Dou, Q.; Ng, K.M. Synthesis of various metal stearates and the corresponding monodisperse metal oxide nanoparticles. Powder Technol. 2016, 301, 949–958. [Google Scholar] [CrossRef]
- Corkery, R.W. Artificial Biomineralisation and Metallic Soaps; Australian National University: Canberra, Australia, 1998. [Google Scholar]
- Nora, A.; Szczepanek, A.; Koenen, G. Metallic soaps. In Ullmann’s Encyclopedia of Industrial Chemistry; Lauro, M.F., Ed.; Wiley–VCH: Weinheim, Germany, 2005; pp. 329–332. [Google Scholar]
- Osmond, G. Zinc Soaps: An Overview of Zinc Oxide Reactivity and Consequences of Soap Formation in Oil-Based Paintings. In Metal Soaps in Art; Casadio, F., Noble, P., Hendricks, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 25–46. [Google Scholar]
- Noble, P. A Brief History of Metal Soaps in Paintings from a Conservation Perspective. In Metal Soaps in Art; Casadio, F., Noble, P., Hendricks, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–22. [Google Scholar]
- Raven, L.E.; Bisschoff, M.; Leeuwestein, M.; Geldof, M.; Hermans, J.J.; Stols-Witlox, M.; Keune, K. Delamination Due to Zinc Soap Formation in an Oil Painting by Piet Mondrian (1872–1944). In Metal Soaps in Art; Casadio, F., Noble, P., Hendricks, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 343–358. [Google Scholar]
- Thomas, M.M.; Clouse, J.A.; Longo, J.M. Adsorption of organic compounds on carbonate minerals. 1. Model compounds and their influence on mineral wettability. Chem. Geol. 1993, 109, 201–213. [Google Scholar] [CrossRef]
- Frye, G.C.; Thomas, M.M. Adsorption of organic compounds on carbonate minerals. 2. Extraction of carboxylic acids from recent and ancient carbonates. Chem. Geol. 1993, 109, 215–226. [Google Scholar] [CrossRef]
- Boon, J.J.; Hoogland, F.; Keune, K.; Parkin, H.M. Chemical processes in aged oil paints affecting metal soap migration and aggregation. In Proceedings of the AIC’s 34th Annual Meeting, Providence, RI, USA, 16–19 June 2006; Volume 19, pp. 16–23. [Google Scholar]
- La Nasa, J.; Modugno, F.; Aloisi, M.; Lluveras-Tenorio, A.; Bonaduce, I. Development of a GC/MS method for the qualitative and quantitative analysis of mixtures of free fatty acids and metal soaps in paint samples. Anal. Chim. Acta 2018, 1001. [Google Scholar] [CrossRef]
- Hermans, J.; Helwig, K. The Identification of Multiple Crystalline Zinc Soap Structures Using Infrared Spectroscopy. Appl. Spectrosc. 2020, 74, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Palacios-beas, E. Infrared spectroscopy of metal carboxylates: II. Analysis of Fe (III), Ni and Zn carboxylate solutions. Hydrometallurgy 2004, 72, 139–148. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Zeleňák, V.; Vargová, Z.; Györyová, K. Correlation of infrared spectra of zinc(II) carboxylates with their structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 262–272. [Google Scholar] [CrossRef]
- Desseyn, H.O. Vibrational analysis of acid derivatives. In The Chemistry of Acid Derivatives. The Chemistry of Functional Groups; Patai, S., Ed.; John Wiley & Sons: Chichester, UK, 1992; Volume 2, Supplement B; pp. 271–304. [Google Scholar]
- Lynch, M.L.; Pan, Y.; Laughlin, R.G. Spectroscopic and Thermal Characterization of 1:2 Sodium Soap/Fatty Acid Acid—Soap Crystals. J. Phys. Chem. 1996, 100, 357–361. [Google Scholar] [CrossRef]
- Lu, Y.; Miller, J.D. Carboxyl stretching vibrations of spontaneously adsorbed and LB-transferred calcium carboxylates as determined by FTIR internal reflection spectroscopy. J. Colloid Interface Sci. 2002, 256, 41–52. [Google Scholar] [CrossRef]
- Hermans, J.J.; Baij, L.; Koenis, M.; Keune, K.; Iedema, P.D. 2D-IR spectroscopy for oil paint conservation: Elucidating the water-sensitive structure of zinc carboxylate clusters in ionomers. Sci. Adv. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, J.; Murphy, A.; Yao, Y.; Yap, G.P.A.; Zumbulyadis, N.; Centeno, S.; Dybowski, C. Coordination geometry of lead carboxylates—Spectroscopic and crystallographic evidence. Dalton Trans. 2015, 44, 2340–2347. [Google Scholar] [CrossRef] [Green Version]
- Cotton, F.A. Modern Coordination Chemistry; Wiley-Interscience: New York, NY, USA, 1960. [Google Scholar]
- Nickolov, Z.; Georgiev, G.; Stoilova, D.; Ivanov, I. Raman and IR study of cobalt acetate dihydrate. J. Mol. Struct. 1995, 354, 119–125. [Google Scholar] [CrossRef]
- Martínez-Casado, F.J.; Rodríguez-Cheda, J.A.; Ramos-Riesco, M.; Redondo-Yélamos, M.I.; Cucinotta, F.; Fernández-Martínez, A. Physicochemistry of Pure Lead(II) Soaps: Crystal Structures, Solid and Liquid Mesophases, and Glass Phases—Crystallographic, Calorimetric, and Pair Distribution Function Analysis. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019; pp. 227–239. [Google Scholar]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. Toward a Complete Molecular Model for the Formation of Metal Soaps in Oil Paints. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019; pp. 47–67. [Google Scholar]
- Corkery, R.W. Langmuir-Blodgett (L-B) multilayer films. Langmuir 1997, 13, 3591–3594. [Google Scholar] [CrossRef]
- Passi, S.; Picardo, M.; De Luca, C.; Luca, C.D.; Nazzaro-Porro, M.; Rossi, L.; Rotilio, G. Saturated dicarboxylic acids as products of unsaturated fatty acid oxidation. Biochim. Biophys. Acta Lipids Lipid Metab. 1993, 1168, 190–198. [Google Scholar] [CrossRef]
- Ioakimoglou, E.; Boyatzis, S.; Argitis, P.; Fostiridou, A.; Papapanagiotou, K.; Yannovits, N. Thin-Film Study on the Oxidation of Linseed Oil in the Presence of Selected Copper Pigments. Chem. Mater. 1999, 11, 2013–2022. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F.; Ribechini, E. Organic mass spectrometry in archaeology: Evidence for Brassicaceae seed oil in Egyptian ceramic lamps. J. Mass Spectrom. 2005, 40, 890–898. [Google Scholar] [CrossRef]
- Ménager, M.; Azémard, C.; Vieillescazes, C. Study of Egyptian mummification balms by FT-IR spectroscopy and GC-MS. Microchem. J. 2014, 114, 32–41. [Google Scholar] [CrossRef]
- Lee, J.; Bonaduce, I.; Modugno, F.; Nasa, J.L.; Ormsby, B.; Berg, K.J.v.d. Scientific investigation into the water sensitivity of twentieth century oil paints. Microchem. J. 2018, 138, 282–295. [Google Scholar] [CrossRef]
- Modugno, F.; Di Gianvincenzo, F.; Degano, I.; Werf, I.D.v.d.; Bonaduce, I.; Berg, K.J.v.d. On the influence of relative humidity on the oxidation and hydrolysis of fresh and aged oil paints. Sci. Rep. 2019, 9, 5533. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F.; Giacomelli, M.; Francesconi, S. Characterisation of proteinaceous binders and drying oils in wall painting samples by gas chromatography-mass spectrometry. J. Chromatogr. A 1999, 846, 113–124. [Google Scholar] [CrossRef]
- Bonaduce, I.; Carlyle, L.; Colombini, M.P.; Duce, C.; Ferrari, C.; Ribechini, E.; Selleri, P.; Tiné, M.R. New Insights into the Ageing of Linseed Oil Paint Binder: A Qualitative and Quantitative Analytical Study. PLoS ONE 2012, 7, e49333. [Google Scholar] [CrossRef] [Green Version]
- Dunitz, J.D.; Robertson, J.M. The crystal and molecular structure of certain dicarboxylic acids. Part, I. Oxalic acid dihydrate. J. Chem. Soc. 1944, 142–148. [Google Scholar] [CrossRef]
- Morrison, J.D.; Robertson, J.M. The crystal and molecular structure of certain dicarboxylic acids. Part IV. β-Succinic acid. J. Chem. Soc. 1949, 980–986. [Google Scholar] [CrossRef]
- Morrison, J.D.; Robertson, J.M. The crystal and molecular structure of certain dicarboxylic acids. Part, V. Adipic acid. J. Chem. Soc. 1949, 987–992. [Google Scholar] [CrossRef]
- Morrison, J.D.; Robertson, J.M. The crystal and molecular structure of certain dicarboxylic acids. Part VI. Sebacic acid. J. Chem. Soc. 1949, 993–1001. [Google Scholar] [CrossRef]
- Susi, H. Infrared spectra of crystalline adipic acid and deuterated analogs. Spectrochim Acta 1956, 15, 1063–1071. [Google Scholar] [CrossRef]
- Macgillavry, C.H.; Hoogschagen, G.; Sixma, F.L.J. The crystal structure of glutaric and pimelic acid. Alternation of properties in the series of dicarboxylic acids. Recl. des Trav. Chim. des Pays-Bas 2010, 67, 869–883. [Google Scholar] [CrossRef]
- Kshnyakina, S.I.; Puchkovskaya, G.A. Spectroscopic investigations of crystals of the homologous series of dicarboxylic acids. J. Appl. Spectrosc. 1981, 34, 556–561. [Google Scholar] [CrossRef]
- Nagakura, M.; Ogawa, Y.; Yoshitomi, K. Infrared Spectroscopic Determination of Alkyd Resin Components. J. Jpn. Soc. Colour Mater. 1968, 41, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.K.; Varughese, S.; Ramamurty, U.; Desiraju, G.R. Odd-Even effect in the elastic modulii of α,ω- alkanedicarboxylic acids. J. Am. Chem. Soc. 2013, 135, 8121–8124. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, A.; Schleiden, T.; Migliavacca, J.M.; Melin, T. Monitoring Crystallization Kinetics of Azelaic Acid by in situ FTIR Spectroscopy in Three-Phase Systems. Chem. Eng. Technol. 2003, 26, 881–889. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Huang, K.; Li, S.; Jiang, J.; Xia, J. Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: Preparation and thermal stabilization for PVC. RSC Adv. 2014, 4, 63576–63585. [Google Scholar] [CrossRef]









| Peak Maxima, Wavenumbers (cm−1) 1 | Assignment | Notes | ||
|---|---|---|---|---|
| Monoacids | Octanedioic (Suberic) Acid | Nonanedioic (Azelaic) Acid | ||
| 3600–2800 br | 3037 | 3045 | vOH | Typically, very broad with a vague maximum. |
| 2960–2956 m-w | n.a. | n.a. | vasCH3 | Variable, according to the number of carbons. |
| 2934–2919 s | 2991, 2951, 2941, 2911 | 2977, 2937, 2915 | vasCH2 | Variable, according to the number of carbons; higher-frequency maxima correspond to a lower distance from the COOH group. |
| 2875–2872 w, s | n.a. | n.a. | vsCH3 | Variable, according to the number of carbons. |
| 2851 m-s | 2872, 2855 | 2875, 2858, 2847 | vsCH2 | Variable, according to the number of carbons. |
| 2670, 2565 br | 2759, 2676, 2599, 2534 | 2774, 2698, 2621, 2553 | vO‒H•••O=C (dimer stretching band) | Structured with weak shoulders; more extended in diacids. |
| 1703 vs | 1695 | 1694 | vC=O, acidic | |
| 1472, 1464, 1411 m | 1470, 1410 | 1471, 1411 | δCH2 scissoring | Split into three components (two in diacids); the 1472 and 1464 cm−1 components are better resolved for monoacids C16-C24. |
| 1457–1450 w | n.a. | n.a. | δasCH3 | Contribution is lower for higher carbon-number chains. |
| 1431 m-w | 1426 | 1436 | δipCOH | Relatively broad; often missed due to overlaps. |
| 1372 m-w | δsCH3 (‘umbrella’ vibration) | |||
| 1356–1347 m-w | 1334 | 1360, 1346 | τCH2 | Splitting in progressions for acids in their crystalline state (in room temperature, higher than C10). |
| 1318–1185 m-w | 1360, 1346 | 1317, 1282, 1268, 1254, 1208, 1196 | wCH2 | Splitting in extended progressions for acids in their crystalline state (in room temperature, higher than C10). |
| 1112–1075 m-w | n.o. | 1105, 1098 | vC‒OH | Up-shifted for longer hydrocarbon chains. Weak, or not observed for diacids. |
| 943–935 m, br | 932 | 920 | δoopC‒O‒H•••O=C (bending dimer band) | |
| 795–741, 725–710 w | 796, 725 | 776, 726 | ρCH2 | Stronger in long hydrocarbon chains; the 725 cm−1 peak is doubly split in crystalline monoacids. |
| 690 | 683 | 681 | δoopC‒O‒H | |
| Peak Maxima, Wavenumbers (cm−1) | Assignment | Notes | ||
|---|---|---|---|---|
| Monoacid Metal Soaps | Octanedioate (Suberate) Metal Soaps | Nonanedioate (Azelate) Metal Soaps | ||
| 3600–3300 | 3590, 3521 Zn | 3565, 3521 Zn | vO‒H (cryst. water) | In Ca and higher Zn monoacid salts. Only in zinc diacid salts. |
| 2956 | n.a. | n.a. | vasCH3 | |
| 2940(sh), 2926–2916 | 2942, 2930, 2909 Na 2978, 2939, 2921, 2907 Ca 2939, 2927, 2905 Zn | 2943, 2936, 2921, 2907 Na 2976, 2947, 2921, 2915 Ca 2939, 2927, 291 Zn | vasCH2 | Downshifted (3–5 cm−1) in monoacid salts, for >C16 (Ca, Na) and >C14 (Zn). Structured in diacid salts. |
| 2874–2870 | n.a. | n.a. | vsCH3 | |
| 2856–2850 Na salts 2851 Ca and Zn salts | 2863 Na 2860, 2850 Ca 2867, 2851 Zn | 2858, 2848 Na 2862, 2849 Ca 2867, 2850 Zn | vsCH2 | Downshifted (~5 cm−1) in monoacid Na salts, for >C9. Split into two components in diacid salts |
| 1628 (br) Ca salt 1617–1619 Zn salts C12-C24 | 1607 Zn | 1616 Zn | δH-O‒H (cryst. water) | In Ca and higher Zn monoacid salts. Only in zinc diacid salts. |
| 1560 Na salts 1579, 1542 Ca salts 1551, 1532 Zn salts, C8, C9 1540 (br) Zn salts C10-C24 | 1563 Na 1581, 1544 Ca 1551, 1537 Zn | 1575 Na 1581, 1542 Ca 1556, 1534 Zn | vasCOO- | Singlet for Na and higher Zn salts. Doublet for Ca and lower Zn salts. |
| 1472–1459 Na salts 1473 Ca salts 1467–1464, Zn salts | 1463, 14447, 1432, Na 1468, 1455, 1431, 1411 Ca 1467, 1460, 1450 Zn | 1463, 1405 Na 1468, 1434, 1420 Ca 1468, 1456 Zn | δCH2 scissoring | Single peak, a stable frequency for Ca and higher monoacid Zn salts. Four- or three-fold structuring in diacid salts. |
| - 1458 Zn salts | n.a. | n.a. | δasCH3 | Overlapped in Na salts; undetectable in Ca salts. Detectable as unresolved shoulder in Zn lower salts. |
| 1423 Na salts 1433, 1411 Ca salts 1410, 1399 Zn salts C8, C9 1398 Zn salts C10-C24 | 1416 Na 1436, 1405 Ca 1412, 1399 Zn | 1434 Na 1431, 1411 Ca 1407, 1398 Zn | vsCOO- | Singlet for Na and higher Zn salts. Doublet for Ca and lower Zn salts. |
| - | n.a. | δsCH3 (‘umbrella’ vibration) | Undetectable in most monoacid salts | |
| 1400–1351 Na salts 1380–1287 Ca salts 1375–1260 Zn salts | 1356 Na 1359, 1332 Ca 1366 Zn | 1339 Na 1343, 1318 Ca 1353 Zn | τCH2 | Progressions of 4 or 5 sub-bands in monoacid salts. Singlet or doublet in diacid salts. |
| 1341–1180 Na salts 1278–1190 Ca salts 1250–1045 Zn salts | 1293, 1200 Na 1293, 1261, 1247, 1217, 1201 Ca 1303, 1207 Zn | 1316, 1288, 1273, 1253, 1233, 1195 Na 1286, 1251, 1200 Ca 1285, 1243 Zn | wCH2 | Progressions following the n/2 pattern (n = total carbon atoms) in Na and Ca salts. Sub-bands in Zn salts. |
| 1185–1100 Na 1114 Ca 1067–1105 Zn | 1100 | 1100 | Unassigned | Weak single band; up-shifting with longer carbon chains in monoacid Zn salts. Single band; not observed in Na and Zn C8di salts. |
| 855–849 | δCH3 + vC-C. | Not observed in monoacid zinc and diacid soaps. | ||
| 725–717, 698 721 Ca salts 747, 723 Zn salts | ρCH2 | Doublet for Na and Zn salts; single peak for Ca salts. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filopoulou, A.; Vlachou, S.; Boyatzis, S.C. Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules 2021, 26, 6005. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26196005
Filopoulou A, Vlachou S, Boyatzis SC. Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules. 2021; 26(19):6005. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26196005
Chicago/Turabian StyleFilopoulou, Anna, Sophia Vlachou, and Stamatis C. Boyatzis. 2021. "Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage" Molecules 26, no. 19: 6005. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26196005