Comparison of Bifurcated Halogen with Hydrogen Bonds
Abstract
:1. Introduction
2. Results
2.1. Shared Lone Pair of Single Base
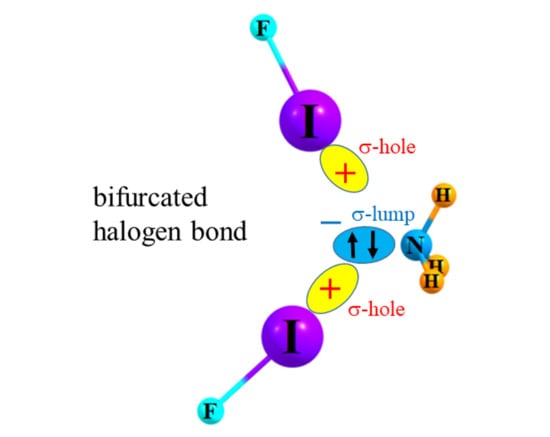
2.2. Shared σ-Hole of Single Acid
2.3. Effects of Complexation on Monomers
2.4. Understanding the Trends
3. Discussion
4. Computational Methods
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1940; p. 450. [Google Scholar]
- Pauling, L.; Corey, R.B. Configurations of polypeptide chains with favored orientations around single bonds: Two new pleated sheets. Proc. Nat. Acad. Sci. USA 1951, 37, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauling, L.; Corey, R.B. Two hydrogen-bonded spiral configurations of the polypeptide chain. J. Am. Chem. Soc. 1950, 72, 5349. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Nat. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; Freeman: San Francisco, CA, USA, 1960. [Google Scholar]
- Sutor, D.J. The C-H...O hydrogen bond in crystals. Nature 1962, 195, 68–69. [Google Scholar] [CrossRef]
- Hamilton, W.C.; Ibers, J.A. Hydrogen Bonding in Solids; W. A. Benjamin: New York, NY, USA, 1968; p. 284. [Google Scholar]
- Vinogradov, S.N.; Linnell, R.H. Hydrogen Bonding; Van Nostrand-Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Joesten, M.D.; Schaad, L.J. Hydrogen Bonding; Marcel Dekker: New York, NY, USA, 1974; p. 622. [Google Scholar]
- Schuster, P.; Zundel, G.; Sandorfy, C. (Eds.) The Hydrogen Bond. Recent Developments in Theory and Experiments; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997; p. 375. [Google Scholar]
- Scheiner, S. Fundamental Features of Hydrogen Bonds. In Pauling’s Legacy—Modern Modelling of the Chemical Bond; Maksic, Z.B., Orville-Thomas, W.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 6, pp. 571–591. [Google Scholar]
- Scheiner, S. Pauling’s Legacy—Modern Modelling of the Chemical Bond; Maksic, Z.B., Orville-Thomas, W.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford: New York, NY, USA, 1999; p. 507. [Google Scholar]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: Oxford, UK, 2009; p. 313. [Google Scholar]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond. Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Chand, A.; Sahoo, D.K.; Rana, A.; Jena, S.; Biswal, H.S. The Prodigious Hydrogen Bonds with Sulfur and Selenium in Molecular Assemblies, Structural Biology, and Functional Materials. Acc. Chem. Res. 2020, 53, 1580–1592. [Google Scholar] [CrossRef]
- Kumar, M.; Francisco, J.S. Evidence of the Elusive Gold-Induced Non-classical Hydrogen Bonding in Aqueous Environments. J. Am. Chem. Soc. 2020, 142, 6001–6006. [Google Scholar] [CrossRef]
- Chand, A.; Biswal, H.S. Hydrogen Bonds with Chalcogens: Looking Beyond the Second Row of the Periodic Table. J. Indian Inst. Sci. 2020, 100, 77–100. [Google Scholar] [CrossRef]
- Mishra, K.K.; Singh, S.K.; Kumar, S.; Singh, G.; Sarkar, B.; Madhusudhan, M.S.; Das, A. Water-Mediated Selenium Hydrogen-Bonding in Proteins: PDB Analysis and Gas-Phase Spectroscopy of Model Complexes. J. Phys. Chem. A 2019, 123, 5995–6002. [Google Scholar] [CrossRef]
- Schmidbaur, H. Proof of Concept for Hydrogen Bonding to Gold, Au⋅⋅⋅H−X. Angew. Chem. Int. Ed. 2019, 58, 5806–5809. [Google Scholar] [CrossRef]
- Wang, P.; Xu, H.-G.; Cao, G.-J.; Zhang, W.-J.; Xu, X.-L.; Zheng, W.-J. Nonconventional Hydrogen Bonds between Silver Anion and Nucleobases: Size-Selected Anion Photoelectron Spectroscopy and Density Functional Calculations. J. Phys. Chem. A 2017, 121, 8973–8981. [Google Scholar] [CrossRef] [PubMed]
- Mundlapati, V.R.; Sahoo, D.K.; Ghosh, S.; Purame, U.K.; Pandey, S.; Acharya, R.; Pal, N.; Tiwari, P.; Biswal, H.S. Spectroscopic Evidences for Strong Hydrogen Bonds with Selenomethionine in Proteins. J. Phys. Chem. Lett. 2017, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of CH···O, SH···O, Chalcogen, and Tetrel Bonds Formed by Neutral and Cationic Sulfur-Containing Compounds. J. Phys. Chem. A 2015, 119, 9189–9199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabardasti, A.; Talebi, N.; Kakanejadifard, A.; Saki, Z. The B–C and C–C bonds as preferred electron source for H-bond and Li-bond interactions in complex pairing of C4B2H6 with HF and LiH molecules. Struct. Chem. 2016, 27, 573–581. [Google Scholar] [CrossRef]
- Møller, K.H.; Hansen, A.S.; Kjaergaard, H.G. Gas Phase Detection of the NH–P Hydrogen Bond and Importance of Secondary Interactions. J. Phys. Chem. A 2015, 119, 10988–10998. [Google Scholar] [CrossRef]
- Viana, R.B.; da Silva, A.B.F. Interaction between PH3 and small water clusters: Understanding the electronic and spectroscopic properties. Comput. Theor. Chem. 2015, 1059, 35–44. [Google Scholar] [CrossRef]
- Sanchez-de-Armas, R.; Ahlquist, M.S.G. On the nature of hydrogen bonds to platinum(II)—Which interaction can predict their strength? Phys. Chem. Chem. Phys. 2015, 17, 812–816. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Comparison of the CH...N and CH...O interactions involving substituted alkanes. J. Mol. Struct. 2000, 552, 17–31. [Google Scholar] [CrossRef]
- Orlova, G.; Scheiner, S. Intermolecular MH...HF bonding in monohydride Mo and W complexes. J. Phys. Chem. A 1998, 102, 260–269. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Comparison of P...D (D=P,N) with other noncovalent bonds in molecular aggregates. J. Chem. Phys. 2011, 135, 184306. [Google Scholar] [CrossRef]
- Grabowski, S.J. A−H…σ Hydrogen Bonds: Dihydrogen and Cycloalkanes as Proton Acceptors. ChemPhysChem 2019, 20, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Martín-Fernández, C.; Montero-Campillo, M.M.; Elguero, J. Hydrogen-Bonding Acceptor Character of Be3, the Beryllium Three-Membered Ring. J. Phys. Chem. A 2018, 122, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Chung, W.-C.; Ley, R.M.; Lin, K.-Y.; Francisco, J.S.; Negishi, E.-I. Molecularly Tuning the Radicaloid N–H···O═C Hydrogen Bond. J. Phys. Chem. A 2016, 120, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Nikpour, M.; Bauzá, A.; Frontera, A. On the Importance of C-H/π and C-H...H-C Interactions in the Solid State Structure of 15-Lipoxygenase Inhibitors Based on Eugenol Derivatives. ChemPhysChem 2015, 16, 2260–2266. [Google Scholar] [CrossRef]
- Grabowski, S.J. Dihydrogen bond and X–H…σ interaction as sub-classes of hydrogen bond. J. Phys. Org. Chem. 2013, 26, 452–459. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Kou, H.; Li, R.; Li, W.-Z.; Cheng, J.-B. Is a Hm-1X (X = O, S, Se, m = 1; X = N, m = 2; X = C, m = 3) radical a better proton donor than HmX–H in hydrogen bonding? Comput. Theor. Chem. 2011, 976, 83–87. [Google Scholar] [CrossRef]
- Kar, T.; Bettinger, H.F.; Scheiner, S.; Roy, A.K. Noncovalent π−π Stacking and CH---π Interactions of Aromatics on the Surface of Single-Wall Carbon Nanotubes: An MP2 Study. J. Phys. Chem. C 2008, 112, 20070–20075. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O.; Versichel, W. Geometry of the N-H...O=C hydrogen bond. 2. Three-center (“bifurcated”) and four-center (“trifurcated”) bonds. J. Am. Chem. Soc. 1984, 106, 244–248. [Google Scholar] [CrossRef]
- Li, A.Y. Theoretical study of linear and bifurcated H-bonds in the systems Y...H2CZn (n = 1, 2; Z = O, S, Se, F, Cl, Br; Y = Cl−, Br−). J. Mol. Struct. (Theochem.) 2008, 862, 21–27. [Google Scholar] [CrossRef]
- Blanco, S.; Lopez, J.C.; Lesarri, A.; Caminati, W.; Alonso, J.L. Bifurcated CH2...O and (C-H)2...F-C weak hydrogen bonds: The oxirane-difluoromethane complex. ChemPhysChem 2004, 5, 1779–1782. [Google Scholar] [CrossRef]
- Cense, J.M.; Agafonov, V.; Ceolin, R.; Ladure, P.; Rodier, N. Crystal and molecular structure analysis of flutamide. Bifurcated helicoidal C-H...O hydrogen bonds. Struct. Chem. 1994, 5, 79–84. [Google Scholar] [CrossRef]
- Duan, X.; Scheiner, S. Behavior of interaction energy and intramolecular bond stretch in linear and bifurcated hydrogen bonds. Int. J. Quantum Chem. QBS 1993, 20, 181–190. [Google Scholar] [CrossRef]
- van Hensbergen, B.; Block, R.; Jansen, L. Effect of direct and indirect exchange interactions on geometries and relative stabilities of H2O and H2S dimers in bifurcated, cyclic, and linear configurations. J. Chem. Phys. 1982, 76, 3161–3168. [Google Scholar] [CrossRef]
- Marsden, C.J.; Smith, B.J.; Pople, J.A.; Schaefer, H.F.; Radom, L. Characterization of the bifurcated structure of the water dimer. J. Chem. Phys. 1991, 95, 1825–1828. [Google Scholar] [CrossRef]
- Thomas, J.; Liu, X.; Jäger, W.; Xu, Y. Unusual H-Bond Topology and Bifurcated H-bonds in the 2-Fluoroethanol Trimer. Angew. Chem. Int. Ed. 2015, 54, 11711–11715. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suryaprakash, N. Intramolecular hydrogen bonds involving organic fluorine in the derivatives of hydrazides: An NMR investigation substantiated by DFT based theoretical calculations. Phys. Chem. Chem. Phys. 2015, 17, 15226–15235. [Google Scholar] [CrossRef]
- Preimesberger, M.R.; Majumdar, A.; Aksel, T.; Sforza, K.; Lectka, T.; Barrick, D.; Lecomte, J.T.J. Direct NMR Detection of Bifurcated Hydrogen Bonding in the α-Helix N-Caps of Ankyrin Repeat Proteins. J. Am. Chem. Soc. 2015, 137, 1008–1011. [Google Scholar] [CrossRef] [Green Version]
- Fulara, A.; Dzwolak, W. Bifurcated hydrogen bonds stabilize fibrils of poyl(L-glutamic) acid. J. Phys. Chem. B 2010, 114, 8278–8283. [Google Scholar] [CrossRef]
- Quinonero, D. Sigma-hole carbon-bonding interactions in carbon-carbon double bonds: An unnoticed contact. Phys. Chem. Chem. Phys. 2017, 19, 15530–15540. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Vessally, E.; Solimannejad, M. Symmetric bifurcated halogen bonds: Substituent and cooperative effects. Mol. Phys. 2016, 114, 3610–3619. [Google Scholar] [CrossRef]
- Novak, M.; Foroutan-Nejad, C.; Marek, R. Asymmetric bifurcated halogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 6440–6450. [Google Scholar] [CrossRef]
- Oliveira, B.G.d.; Araújo, R.d.C.M.U.d.; Leite, E.S.; Ramos, M.N. A theoretical analysis of topography and molecular parameters of the CFCl3···O3 complex: Linear and bifurcate halogen-oxygen bonding interactions. Int. J. Quantum Chem. 2011, 111, 111–116. [Google Scholar] [CrossRef]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η2(Cl–Pt). Cryst. Growth Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-involving halogen bond Ar–I⋯[dz2PtII] in a platinum acetylacetonate complex. CrystEngComm 2020, 22, 554–563. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Kirina, Y.V.; Kukushkin, V.Y. Halogen bonding between metal centers and halocarbons. Chem. Commun. 2016, 52, 5565–5568. [Google Scholar] [CrossRef] [Green Version]
- Bartashevich, E.; Troitskaya, E.; Pendás, Á.M.; Tsirelson, V. Understanding the bifurcated halogen bonding N...Hal...N in bidentate diazaheterocyclic compounds. Comput. Theor. Chem. 2015, 1053, 229–237. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F.; Vessally, E. An ab initio study on substituent and cooperative effects in bifurcated fluorine bonds. Mol. Phys. 2017, 115, 278–287. [Google Scholar] [CrossRef]
- Massahi, S.; Ghobadi, M.; Nikoorazm, M. Exceptional bifurcated chalcogen bonding interaction between Ph2N2O2 and only one σ–hole on XCY (X = S, Se, Te and Y = O, S, Se, Te): A DFT study. Theor. Chem. Acc. 2020, 139, 162. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. Bifurcated chalcogen bonds: A theoretical study on the structure, strength and bonding properties. Chem. Phys. Lett. 2015, 634, 210–215. [Google Scholar] [CrossRef]
- Daolio, A.; Scilabra, P.; Di Pietro, M.E.; Resnati, C.; Rissanen, K.; Resnati, G. Binding motif of ebselen in solution: Chalcogen and hydrogen bonds team up. New J. Chem. 2020, 44, 20697–20703. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. Substituent effects on geometry and bonding properties of asymmetric bifurcated pnicogen bonds: A theoretical study. Chem. Phys. Lett. 2016, 650, 52–56. [Google Scholar] [CrossRef]
- Grabowski, S.J. Bifurcated Triel Bonds—Hydrides and Halides of 1,2-Bis(Dichloroboryl)Benzene and 1,8-Bis(Dichloroboryl)Naphthalene. Crystal 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Esrafili, M.D.; Sadr-Mousavi, A. A computational study on the strength and nature of bifurcated aerogen bonds. Chem. Phys. Lett. 2018, 698, 1–6. [Google Scholar] [CrossRef]
- Zhao, D.-X.; Yang, Z.-Z. Investigation of the distinction between van der Waals interaction and chemical bonding based on the PAEM-MO diagram. J. Comput. Chem. 2014, 35, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.; Matveychuk, Y.; Tsirelson, V. Identification of the Tetrel Bonds between Halide Anions and Carbon Atom of Methyl Groups Using Electronic Criterion. Molecules 2019, 24, 1083. [Google Scholar] [CrossRef] [Green Version]
- Bartashevich, E.V.; Yushina, I.D.; Stash, A.I.; Tsirelson, V.G. Halogen Bonding and Other Iodine Interactions in Crystals of Dihydrothiazolo(oxazino)quinolinium Oligoiodides from the Electron-Density Viewpoint. Cryst. Growth Des. 2014, 14, 5674–5684. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Liew, J.Y.; Beer, P.D. Thermodynamics of Anion Binding by Chalcogen Bonding Receptors. Chem. Eur. J. 2018, 24, 14560–14566. [Google Scholar] [CrossRef]
- Scheiner, S. Differential Binding of Tetrel-Bonding Bipodal Receptors to Monatomic and Polyatomic Anions. Molecules 2019, 24, 227. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. Tetrel Bonding as a Vehicle for Strong and Selective Anion Binding. Molecules 2018, 23, 1147. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S.; Michalczyk, M.; Zierkiewicz, W. Coordination of anions by noncovalently bonded σ-hole ligands. Coord. Chem. Rev. 2020, 405, 213136. [Google Scholar] [CrossRef]
- Scheiner, S.; Michalczyk, M.; Wysokiński, R.; Zierkiewicz, W. Structures and energetics of clusters surrounding diatomic anions stabilized by hydrogen, halogen, and other noncovalent bonds. Chem. Phys. 2020, 530, 110590. [Google Scholar] [CrossRef]
- Scheiner, S.; Michalczyk, M.; Zierkiewicz, W. Structures of clusters surrounding ions stabilized by hydrogen, halogen, chalcogen, and pnicogen bonds. Chem. Phys. 2019, 524, 55–62. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Zhou, Y.; Sun, H.-Y.; Yu, Z.-W. Comparative Study of Halogen- and Hydrogen-Bond Interactions between Benzene Derivatives and Dimethyl Sulfoxide. ChemPhysChem 2015, 16, 2594–2601. [Google Scholar] [CrossRef]
- Tafipolsky, M. Challenging Dogmas: Hydrogen Bond Revisited. J. Phys. Chem. A 2016, 120, 4550–4559. [Google Scholar] [CrossRef]
- Kolár, M.; Hostaš, J.; Hobza, P. The strength and directionality of a halogen bond are co-determined by the magnitude and size of the s-hole. Phys. Chem. Chem. Phys. 2014, 16, 9987–9996. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Wakisaka, A.; Ono, T.; Sonoda, T. Magnitude and origin of the attraction and directionality of the halogen bonds of the complexes of C6F5X and C6H5X (X = I, Br, Cl and F) with Pyridine. Chem. Eur. J. 2012, 18, 951–960. [Google Scholar] [CrossRef]
- Shields, Z.P.; Murray, J.S.; Politzer, P. Directional tendencies of halogen and hydrogen bonds. Int. J. Quantum Chem. 2010, 110, 2823–2832. [Google Scholar] [CrossRef]
- Riley, K.E.; Merz, K.M. Insights into the strength and origin of halogen bonding: The halobenzene-formaldehyde dimer. J. Phys. Chem. A 2007, 111, 1688–1694. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. The relative roles of electrostatics and dispersion in the stabilization of halogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 17742–17751. [Google Scholar] [CrossRef]
- Riley, K.E. Critical comparison of RX...Y and RH...Y directionality in halogen and hydrogen bonds using modern computational chemistry methods. Chem. Phys. Lett. 2020, 744, 137221. [Google Scholar] [CrossRef]
- Hill, J.G.; Legon, A.C. On the directionality and non-linearity of halogen and hydrogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdawy, A.E.; Murray, J.S.; Politzer, P.; Bleiziffer, P.; Heßelmann, A.; Görling, A.; Clark, T. Directional noncovalent interactions: Repulsion and dispersion. J. Chem. Theory Comput. 2013, 9, 2264–2275. [Google Scholar] [CrossRef]
- Stone, A.J. Are halogen bonded structures electrostatically driven? J. Am. Chem. Soc. 2013, 135, 7005–7009. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Sensitivity of pnicogen, chalcogen, halogen and H-bonds to angular distortions. Chem. Phys. Lett. 2012, 532, 31–35. [Google Scholar] [CrossRef]
- Riley, K.E.; Vazquez, M.; Umemura, C.; Miller, C.; Tran, K.-A. Exploring the (Very Flat) Potential Energy Landscape of R−Br⋅⋅⋅π Interactions with Accurate CCSD(T) and SAPT Techniques. Chem. Eur. J. 2016, 22, 17690–17695. [Google Scholar] [CrossRef] [Green Version]
- Kirshenboim, O.; Kozuch, S. How to Twist, Split and Warp a σ-Hole with Hypervalent Halogens. J. Phys. Chem. A 2016, 120, 9431–9445. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Cramer, C.J.; Huber, S.M. Hypervalent Iodine(III) Compounds as Biaxial Halogen Bond Donors. J. Am. Chem. Soc. 2020, 142, 8633–8640. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Wysokiński, R.; Michalczyk, M.; Scheiner, S. Chalcogen bonding of two ligands to hypervalent YF4 (Y = S, Se, Te, Po). Phys. Chem. Chem. Phys. 2019, 21, 20829–20839. [Google Scholar] [CrossRef]
- Michalczyk, M.; Zierkiewicz, W.; Wysokiński, R.; Scheiner, S. Hexacoordinated Tetrel-Bonded Complexes between TF4 (T = Si, Ge, Sn, Pb) and NCH: Competition between σ- and π-Holes. ChemPhysChem 2019, 20, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S.; Lu, J. Halogen, Chalcogen, and Pnicogen Bonding Involving Hypervalent Atoms. Chem. Eur. J. 2018, 24, 8167–8177. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of halide receptors based on H, halogen, chalcogen, pnicogen, and tetrel bonds. Faraday Disc. 2017, 203, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assembly of Effective Halide Receptors from Components. Comparing Hydrogen, Halogen, and Tetrel Bonds. J. Phys. Chem. A 2017, 121, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Highly Selective Halide Receptors Based on Chalcogen, Pnicogen, and Tetrel Bonds. Chem. Eur. J. 2016, 22, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.; Scheiner, S. Building a Better Halide Receptor: Optimum Choice of Spacer, Binding Unit, and Halosubstitution. ChemPhysChem 2016, 17, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Infor. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Orlova, A.P.; Jasien, P.G. Halogen bonding in self-assembling systems: A comparison of intra- and interchain binding energies. Comput. Theor. Chem. 2018, 1139, 63–69. [Google Scholar] [CrossRef]
- Forni, A.; Pieraccini, S.; Franchini, D.; Sironi, M. Assessment of DFT Functionals for QTAIM Topological Analysis of Halogen Bonds with Benzene. J. Phys. Chem. A 2016, 120, 9071–9080. [Google Scholar] [CrossRef]
- Nziko, V.d.P.N.; Scheiner, S. Chalcogen Bonding between Tetravalent SF4 and Amines. J. Phys. Chem. A 2014, 118, 10849–10856. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Vessally, E. A theoretical evidence for cooperative enhancement in aerogen-bonding interactions: Open-chain clusters of KrOF2 and XeOF2. Chem. Phys. Lett. 2016, 662, 80–85. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Scheiner, S. Comparison of tetrel bonds in neutral and protonated complexes of pyridineTF3 and furanTF3 (T = C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. Systematic Elucidation of Factors That Influence the Strength of Tetrel Bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauzá, A.; Alkorta, I.; Frontera, A.; Elguero, J. On the Reliability of Pure and Hybrid DFT Methods for the Evaluation of Halogen, Chalcogen, and Pnicogen Bonds Involving Anionic and Neutral Electron Donors. J. Chem. Theory Comput. 2013, 9, 5201–5210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georg, H.C.; Fileti, E.E.; Malaspina, T. Ab initio study of weakly bound halogen complexes: RX...PH3. J. Mol. Model. 2013, 19, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Scheiner, S. Effects of Halogen, Chalcogen, Pnicogen, and Tetrel Bonds on IR and NMR Spectra. Molecules 2019, 24, 2822. [Google Scholar] [CrossRef] [Green Version]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Brauer, B.; Kesharwani, M.K.; Martin, J.M.L. Some Observations on Counterpoise Corrections for Explicitly Correlated Calculations on Noncovalent Interactions. J. Chem. Theory Comput. 2014, 10, 3791–3799. [Google Scholar] [CrossRef]
- Latajka, Z.; Scheiner, S. Primary and secondary basis set superposition error at the SCF and MP2 levels: H3N--Li+ and H2O--Li+. J. Chem. Phys. 1987, 87, 1194–1204. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Keith, T.A. AIMALL; TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]










| Base | Acid | R | θ | -Eint | ρBCP | E(2) |
|---|---|---|---|---|---|---|
| NH3 | 1 FBr | 2.339 | 16.30 | 560 | 51.10 | |
| 2 FBr | 2.760 | 79.7 | 15.60 | 207 | 8.95 | |
| 1 FBr(def) a | 9.43 | 213 | 10.71 | |||
| NH3 | 1 FI | 2.493 | 19.17 | 465 | 43.61 | |
| 2 FI | 2.730 | 85.7 | 17.51 | 263 | 14.65 | |
| 1 FI(def) | 11.63 | 267 | 16.37 | |||
| NH3 | 1 FH | 1.672 | 13.88 | 534 | 40.09 | |
| 2 FH | 1.895 | 66.2 | 13.83 | 290 | 13.85 | |
| 1 FH(def) | 9.73 | 290 | 15.44 | |||
| NCH | 1 FBr | 2.629 | 7.20 | 247 | 12.13 | |
| 2 FBr | 2.766 | 88.2 | 10.51 | 178 | 5.68 | |
| 1 FBr(def) | 6.07 | 179 | 6.33 | |||
| NCH | 1 FI | 2.621 | 10.20 | 301 | 20.09 | |
| 2 FI | 2.868 | 91.8 | 13.48 | 173 | 6.66 | |
| 1 FI(def) | 8.05 | 175 | 7.73 | |||
| NCH | 1 FH | 1.875 | 7.32 | 271 | 12.29 | |
| 2 FH | 1.974 | 85.4 | 9.37 | 205 | 5.89 | |
| 1 FH(def) | 6.04 | 205 | 6.51 |
| Acid | Base | R | θ | -Eint | ρBCP | E(2) |
|---|---|---|---|---|---|---|
| FBr | 1 NCH | 2.629 | 7.20 | 247 | 12.13 | |
| 2 NCH | 3.090 | 60.8 | 4.31 | 111 | 1.86 | |
| 1 NCH (def) a | 3.33 | 112 | 1.93 | |||
| FI | 1 NCH | 2.621 | 10.20 | 301 | 20.09 | |
| 2 NCH | 3.081 | 59.0 | 6.83 | 139 | 3.74 | |
| 1 NCH (def) | 5.17 | 139 | 3.96 | |||
| FH | 1 NCH | 1.875 | 7.32 | 271 | 12.29 | |
| 2 NCH | 2.207 | 90.3 | 6.46 | 144 | 2.87 | |
| 1 NCH (def) | 4.66 | 147 | 2.32 |
| Base | Acid | Δr | Δν(FX) | Δσ(X) | Δσ(F) | Δσ(N) |
|---|---|---|---|---|---|---|
| NH3 | 1 FBr | 0.061 | −107.2 | 1670.5 | −790.8 | −17.6 |
| 2 FBr | 0.012 | −27.0 | 687.5 | −168.0 | −4.3 | |
| NH3 | 1 FI | 0.055 | −48.5 | 71.5 | −421.3 | −7.0 |
| 2 FI | 0.013 | +8.1 | 37.7 | −214.1 | +10.3 | |
| NH3 | 1 FH | 0.036 | −884.8 | −7.4 | −35.9 | −8.8 |
| 2 FH | 0.010 | −275.6 | −3.0 | −21.7 | −10.6 | |
| NCH | 1 FBr | 0.013 | −29.1 | 680.6 | −183.8 | −21.6 |
| 2 FBr | 0.006 | −8.7 | 484.5 | −122.0 | −12.8 | |
| NCH | 1 FI | 0.026 | −26.2 | 48.8 | −297.0 | −12.7 |
| 2 FI | 0.008 | +15.4 | 30.4 | −173.1 | −2.6 | |
| NCH | 1 FH | 0.008 | −235.5 | −2.2 | −6.9 | −19.4 |
| 2 FH | 0.005 | −130.1 | −1.6 | −11.9 | −21.6 | |
| 1 NCH | FBr | 0.013 | −29.1 | 680.6 | −183.8 | −21.6 |
| 2NCH | 0.013 | −20.5 | 78.2 | −66.5 | −45.2 | |
| 1 NCH | FI | 0.026 | −26.2 | 48.8 | −297.0 | −12.7 |
| 2NCH | 0.020 | −20.7 | 16.3 | −136.1 | −41.1 | |
| 1 NCH | FH | 0.008 | −235.5 | −2.2 | −6.9 | −19.4 |
| 2NCH | 0.009 | −182.7 | −1.3 | −16.3 | −41.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiner, S. Comparison of Bifurcated Halogen with Hydrogen Bonds. Molecules 2021, 26, 350. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020350
Scheiner S. Comparison of Bifurcated Halogen with Hydrogen Bonds. Molecules. 2021; 26(2):350. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020350
Chicago/Turabian StyleScheiner, Steve. 2021. "Comparison of Bifurcated Halogen with Hydrogen Bonds" Molecules 26, no. 2: 350. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020350