4. Experimental Part
General
NMR spectra
1H and
13C were measured in solutions of CDCl
3 or DMSO-
d6 on Bruker AVANCE-III HD instrument (at 400.40 or 100.61 MHz, respectively). HRMS spectra were measured in MeCN solutions on Bruker maXis impact (electrospray ionization, employing HCO
2Na–HCO
2H for calibration). See
Supplementary Materials for NMR (S2–S97) and HRMS (S98–S114) spectral charts. IR spectra was measured on FT-IR spectrometer Shimadzu IRAffinity-1S equipped with an ATR sampling module. Reaction progress, purity of isolated compounds, and R
f values were assessed by TLC on Silufol UV-254 plates. Column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Melting points were measured on Stuart SMP30 apparatus. All reagents and solvents were purchased from commercial vendors and used as received.
Preparation of 3-(2-nitroethyl)-1H-indoles 4 (general procedure): A 5-mL round-bottom flask was charged with indole 1 (2.0 mmol), β-nitrostyrene 2 (2.1 mmol), acetic acid (10 μL) and ethanol (1 mL). The mixture was refluxed for 2–8 h, while the reaction progress was monitored by TLC. After complete consumption of the starting material, the mixture was cooled down to room temperature, and the resulting precipitate was collected by filtration. Alternatively, the reaction mixture was concentrated in vacuo, and the residue was purified by preparative column chromatography (eluent EtOAc/Hex 1:4).
3-(1-(2-Methoxyphenyl)-2-nitroethyl)-2-methyl-1H-indole (
4ad): bright yellow solid, mp (EtOH) 145.3–146.7 °C (Literature data: mp 128–129 °C [
30]) R
f 0.38 (EtOAc/Hex, 1:4). Yield: 521 mg (1.68 mmol, 84%).
1H NMR (400 MHz, Chloroform-
d) δ 7.85 (s, 1H), 7.57–7.51 (m, 1H), 7.36–7.30 (m, 1H), 7.30–7.19 (m, 2H), 7.15–7.03 (m, 2H), 6.92–6.84 (m, 2H), 5.49–5.42 (m, 1H), 5.27–5.19 (m, 1H), 5.16–5.08 (m, 1H), 3.88 (s, 3H), 2.46–2.39 (m, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 157.0, 135.5, 133.4, 128.6, 128.5, 127.54, 127.47, 121.2, 120.7, 119.7, 119.1, 110.8, 110.7, 108.2, 77.7, 55.5, 35.8, 12.2. IR, v
max/cm
−1: 3451, 2998, 1549, 1253, 1024, 737. HRMS (ES TOF) calculated for (M + Na)
+ C
18H
18N
2NaO
3 333.1210, found 333.1205 (1.5 ppm).
3-(2-Nitro-1-phenylethyl)-2-phenyl-1H-indole (
4ba): colorless solid, mp (EtOH) 142–143.2 °C (Literature data: mp 142.2–143.4 °C [
18]), R
f 0.63 (EtOAc/Hex, 1:4). Yield: 657 mg (1.92 mmol, 96%).
1H NMR (400 MHz, DMSO-
d6) δ 11.46 (s, 1H), 7.66 (d,
J = 8.1 Hz, 1H), 7.54 (d,
J = 3.5 Hz, 4H), 7.46 (m,
J = 6.6, 3.2 Hz, 1H), 7.38 (d,
J = 8.1 Hz, 1H), 7.34–7.26 (m, 4H), 7.21 (t,
J = 6.8 Hz, 1H), 7.11 (t,
J = 7.6 Hz, 1H), 6.98 (t,
J = 7.5 Hz, 1H), 5.59–5.41 (m, 2H), 5.21 (t,
J = 8.2 Hz, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 140.3, 136.6, 136.3, 132.4, 128.9 (2C), 128.74 (2C), 128.69 (2C), 128.2, 127.3 (2C), 126.8, 126.3, 121.6, 120.0, 119.3, 111.7, 108.7, 78.2, 40.3. IR, v
max/cm
−1: 3417, 3046, 1747, 1699, 1684, 1504, 1489, 1376, 1243. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
18N
2NaO
2 365.1260, found 365.1253 (2.1 ppm).
3-(2-Nitro-1-(p-tolyl)ethyl)-2-phenyl-1H-indole (
4bb): colorless solid, mp (EtOH) 159.8–161.4 °C (Literature data: mp 158–160 °C [
31]), R
f 0.48 (EtOAc/Hex, 1:4). Yield: 606 mg (1.7 mmol, 85%).
1H NMR (400 MHz, Chloroform-
d) 8.15 (s, 1H), 7.54 (d,
J = 8.0 Hz, 1H), 7.50–7.35 (m, 6H), 7.27–7.18 (m, 3H), 7.15–7.06 (m, 3H), 5.29 (t,
J = 8.0 Hz, 1H), 5.21–5.07 (m, 2H), 2.32 (s, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 137.3, 137.27, 137.2, 136.5, 132.6, 130.0 (2C), 129.4 (2C), 129.2 (2C), 129.0, 127.8 (2C), 127.5, 122.9, 120.7, 120.5, 111.8, 110.1, 79.6, 40.9, 21.5. IR, v
max/cm
−1: 3403, 1545, 1511, 1455, 1427, 1378, 1243, 1188, 1067, 1022. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
20N
2NaO
2 379.1417, found 379.1418 (−0.2 ppm).
3-(1-(4-Isopropylphenyl)-2-nitroethyl)-2-phenyl-1H-indole (4bc): yellowish solid, mp (EtOH) 71.1–72.6 °C, Rf 0.63 (EtOAc/Hex, 1:4). Yield: 515 mg (1.34 mmol, 67%). 1H NMR (400 MHz, DMSOd6) δ 11.44 (s, 1H), 7.68 (d, J = 7.9 Hz, 1H), 7.56–7.53 (m, 4H), 7.46 (m, J = 8.6, 6.2, 2.6 Hz, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.23 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 8.3 Hz, 2H), 7.11 (m, J = 8.1, 7.6, 1.1 Hz, 1H), 6.98 (ddd, J = 8.1, 7.0, 1.1 Hz, 1H), 5.57–5.39 (m, 2H), 5.18 (dd, J = 9.0, 7.5 Hz, 1H), 2.81 (p, J = 6.9 Hz, 1H), 1.14 (dd, J = 6.9, 0.8 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 146.8, 137.6, 136.5, 136.2, 132. 4, 128.8 (2C), 128.7 (2C), 128.2, 127.2 (2C), 126.6 (2C), 126.3, 121.5, 120.1, 119.3, 111.7, 108.8, 78.3, 40.0, 33.0, 23.84, 23.82. IR, vmax/cm−1: 2964, 2872, 1740, 1653, 1455, 1373, 1308, 1246, 1045. HRMS (ES TOF) calculated for (M + Na)+ C25H24N2NaO2 407.1730, found 407.1718 (3.0 ppm).
3-(1-(2-Methoxyphenyl)-2-nitroethyl)-2-phenyl-1H-indole (4bd): pale yellow solid, mp (EtOH) 195.2–197.9 °C, Rf 0.44 (EtOAc/Hex, 1:3). Yield: 446 mg (1.2 mmol, 60%). 1H NMR (400 MHz, Chloroform-d) δ 8.15 (s, 1H), 7.66 (d, J = 7.9 Hz, 1H), 7.48–7.42 (m, 4H), 7.42–7.34 (m, 3H), 7.29–7.20 (m, 2H), 7.18–7.13 (m, 1H), 6.93–6.82 (m, 2H), 5.62 (dd, J = 8.9, 6.5 Hz, 1H), 5.18 (d, J = 1.3 Hz, 1H), 5.16 (d, J = 3.6 Hz, 1H), 3.78 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 156.9, 137.2, 136.2, 132.7, 129.3, 128.9 (2C), 128.8 (2C), 128.7, 128.5, 127.84, 127.76, 122.4, 120.8, 120.4, 120.3, 111.5, 110.6, 109.2, 77.7, 55.4, 36.3. IR, vmax/cm−1: 3408, 2935, 1547, 1238, 1026, 744. HRMS (ES TOF) calculated for (M + Na)+ C23H20N2NaO3 395.1366, found 395.1351 (3.7 ppm).
3-(1-(4-Methoxyphenyl)-2-nitroethyl)-2-phenyl-1H-indole (
4be): colorless solid, mp (EtOH) 145.0–147.1 °C (Literature data: mp 156–157 °C [
32]) R
f 0.37 (EtOAc/Hex, 1:4). Yield: 632 mg (1.7 mmol, 85%).
1H NMR (400 MHz, Chloroform-
d) δ 8.19 (br. s, 1H), 7.57 (d,
J = 8.0 Hz, 1H), 7.53–7.38 (m, 6H), 7.33–7.21 (m, 3H), 7.15 (t,
J = 7.6 Hz, 1H), 6.89–6.82 (m, 2H), 5.30 (t,
J = 7.9 Hz, 1H), 5.21–5.09 (m, 2H), 3.80 (s, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 158.7, 137.0, 136.2, 132.3, 132.0, 129.1 (2C), 128.9 (2C), 128.72, 128.70 (2C), 127.1, 122.6, 120.4, 120.1, 114.3 (2C), 111.5, 109.9, 79.5, 55.4, 40.3. IR, v
max/cm
−1: 3388, 3055, 2959, 1609, 1539, 1513, 1462, 1428, 1378, 1241, 1183. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
20N
2NaO
3 395.1366, found 395.1356 (2.7 ppm).
3-(1-(2-Fluorophenyl)-2-nitroethyl)-2-phenyl-1H-indole (4bf): colorless solid, mp (EtOH) 154.2–157.4 °C, Rf 0.45 (EtOAc/Hex, 1:4). Yield: 598 mg (1.66 mmol, 83%). 1H NMR (400 MHz, Chloroform-d) δ 8.17 (s, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.44–7.37 (m, 7H), 7.26–7.17 (m, 2H), 7.13 (t, J = 7.5 Hz, 1H), 7.10–6.97 (m, 2H), 5.55 (t, J = 8.0 Hz, 1H), 5.26–5.03 (m, 2H).13C NMR (101 MHz, Chloroform-d) δ 160.5 (d, J = 246.7 Hz), 137.3, 136.0, 132.1, 129.5 (d, J = 3.7 Hz), 129.2 (d, J = 8.4 Hz), 129.0 (2C), 128.8 (2C), 128.7, 127.1, 126.4 (d, J = 13.8 Hz), 124.5 (d, J = 3.6 Hz), 122.5, 120.4, 119.8, 115.9 (d, J = 22.2 Hz), 111.5, 107.9, 77.3 (d, J = 2.8 Hz), 35.6 (d, J = 1.9 Hz). IR, vmax/cm−1: 3408, 1552, 1482, 1460, 1431, 1378, 1316, 1096. HRMS (ES TOF) calculated for (M + Na)+ C22H17FN2NaO2 383.1166, found 383.1168 (−0.5 ppm).
3-(1-(4-Fluorophenyl)-2-nitroethyl)-2-phenyl-1H-indole (4bg): colorless solid, mp (EtOH) 145.0–147.1 °C, Rf 0.46 (EtOAc/Hex, 1:4). Yield: 655 mg (1.82 mmol, 91%). 1H NMR (400 MHz, Chloroform-d) δ 8.19 (s, 1H), 7.60–7.38 (m, 7H), 7.34–7.21 (m, 3H), 7.14 (t, J = 7.5 Hz, 1H), 6.99 (t, J = 8.5 Hz, 2H), 5.30 (t, J = 7.9 Hz, 1H), 5.23–5.03 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 162.0 (d, J = 246.3 Hz), 137.1, 136.1, 135.8 (d, J = 3.3 Hz), 132.2, 129.2 (d, J = 8.0 Hz, 2C), 129.2 (2C), 128.9 (3C), 127.0, 122.7, 120.6, 119.9, 115.9 (d, J = 21.5 Hz, 2C), 111.6, 109.5, 79.2, 40.3. IR, vmax/cm−1: 3397, 3046, 1549, 1501, 1455, 1428, 1378, 1320, 1245, 1219. HRMS (ES TOF) calculated for (M + Na)+ C22H17FN2NaO2 383.1166, found 383.1174 (-2.1 ppm).
3-(1-(4-Chlorophenyl)-2-nitroethyl)-2-phenyl-1H-indole (
4bh): colorless solid, mp (EtOH) 145.6–147.1 °C (Literature data: mp 118–123.2 °C [
20]), R
f 0.49 (EtOAc/Hex, 1:4). Yield: 632 mg (1.68 mmol, 84%).
1H NMR (400 MHz, Chloroform-
d) δ 8.22 (s, 1H), 7.55–7.39 (m, 7H), 7.34–7.24 (m, 5H), 7.21–7.14 (m, 1H), 5.32 (t,
J = 7.9 Hz, 1H), 5.23–5.03 (m, 2H).
13C NMR (101 MHz, Chloroform-
d) δ 138.5, 137.2, 136.1, 133.2, 132.1, 129.1 (4C), 129.0 (2C), 128.9, 128.8 (2C), 126.9, 122.7, 120.6, 119.8, 111.7, 109.1, 79.0, 40.4. IR, v
max/cm
−1: 3397, 3041, 1681, 1546, 1487, 1393, 1306, 1250, 1204. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
17ClN
2NaO
2 399.0871, found 399.0875 (-1.2 ppm).
N,N-Dimethyl-4-(2-nitro-1-(2-phenyl-1H-indol-3-yl)ethyl)aniline (4bi): red solid, mp (EtOH) 78.6–80.9 °C, Rf 0.34 (EtOAc/Hex, 1:4). Yield: 724 mg (1.88 mmol, 94%). 1H NMR (400 MHz, Chloroform-d) δ 8.15 (s, 1H), 7.58 (d, J = 8.1 Hz, 1H), 7.48–7.45 (m, 4H), 7.44–7.41 (m, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.25–7.16 (m, 3H), 7.16–7.07 (m, 1H), 6.66 (d, J = 8.9 Hz, 2H), 5.23 (t, J = 7.9 Hz, 1H), 5.12 (d, J = 7.3 Hz, 2H), 2.91 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 149.7, 136.9, 136.2, 132.5, 129.0 (2C), 128.9 (2C), 128.6, 128.4 (2C), 127.5, 127.3, 122.5, 120.4, 120.3, 112.9 (2C), 111.4, 110.2, 79.7, 40.7 (2C), 40.3. IR, vmax/cm−1: 2921, 1740, 1720, 1614, 1523, 1460, 1241, 1169, 1053. HRMS (ES TOF) calculated for (M + H)+ C24H24N3O2 386.1863, found 386.1855 (2.0 ppm).
3-(1-(3,5-Dimethyl-1H-pyrazol-4-yl)-2-nitroethyl)-2-phenyl-1H-indole (
4bj): reddish amorphous, mp 90.5–97.5 °C (Literature data: mp 96–101 °C [
33]), R
f 0.28 (EtOAc/Hex, 1:4). Yield: 426 mg (1.18 mmol, 59%).
1H NMR (400 MHz, Acetone-
d6) δ 10.64 (s, 1H), 7.74 (d,
J = 8.1 Hz, 1H), 7.59–7.41 (m, 6H), 7.16 (ddd,
J = 8.1, 7.1, 1.1 Hz, 1H), 7.09 (ddd,
J = 8.1, 7.1, 1.1 Hz, 1H), 5.50–5.38 (m, 1H), 5.35–5.24 (m, 2H), 2.03 (s, 6H).
13C NMR (101 MHz, Acetone
d6) δ 142.2 (2C), 137.2, 137,0, 133.8, 129.6 (2C), 129.3 (2C), 128.7, 128.0, 122.3, 120.6, 120.0, 114.1, 112.1, 109.9, 78.7, 33.6, 11.9 (2C). IR, v
max/cm
−1: 3398, 1549, 1455, 1424, 1378, 1311, 1159, 1072. HRMS (ES TOF) calculated for (M + H)
+ C
21H
21N
4O
2 361.1659, found 361.1656 (0.9 ppm).
3-(2-Nitro-1-phenylethyl)-2-(p-tolyl)-1H-indole (4ca): colorless solid, mp (EtOH) 151.0–152.5 °C, Rf 0.49 (EtOAc/Hex, 1:4). Yield: 528 mg (1.48 mmol, 74%). 1H NMR (400 MHz, Chloroform-d) δ 8.16 (s, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.46–7.19 (m, 11H), 7.14 (t, J = 7.6 Hz, 1H), 5.35 (t, J = 7.9 Hz, 1H), 5.25–5.11 (m, 2H), 2.45 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 140.0, 138.7, 137.1, 136.0, 129.7 (2C), 129.2, 128.9 (2C), 128.7 (2C), 127.5 (2C), 127.2, 127.1, 122.4, 120.2, 119.9, 111.3, 109.3, 79.1, 40.8, 21.4. IR, vmax/cm−1: 3422, 3051, 2925, 1545, 1489, 1453, 1426, 1364, 1303, 1248, 1166. HRMS (ES TOF) calculated for (M + Na)+ C23H20N2NaO2 379.1417, found 379.1419 (-0.4 ppm).
2-(3,5-Dimethylphenyl)-3-(2-nitro-1-phenylethyl)-1H-indole (4da): yellow solid, mp (EtOH) 68,5–70,4 °C, Rf 0.35 (EtOAc/Hex, 1:4). Yield: 690 mg (1.86 mmol, 93%). 1H NMR (400 MHz, Chloroform-d) δ 8.17 (s, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.44–7.38 (m, 3H), 7.35 (t, J = 7.3 Hz, 2H), 7.30 (s, 1H), 7.27 (s, 2H), 7.25–7.21 (m, 2H), 7.15 (t, J = 8.2 Hz, 1H), 5.38 (t, J = 7.9 Hz, 1H), 5.25 (dd, J = 12.5, 7.6 Hz, 1H), 5.16 (dd, J = 12.4, 8.3 Hz, 1H), 2.36 (d, J = 11.4 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 140.3, 137.43, 137.40, 137.3, 136.0, 130.3, 130.0, 129.7, 129.0 (2C), 127.6 (2C), 127.27, 127.25, 126.2, 122.4, 120.3, 119.9, 111.4, 109.4, 79.2, 41.0, 20.0, 19.8. IR, vmax/cm−1: 3412, 2926, 1552, 1458, 1376, 1311, 1017. HRMS (ES TOF) calculated for (M + Na)+ C24H22N2NaO2 393.1571, found 393.1573 (0.6 ppm).
2-(4-Methoxyphenyl)-3-(2-nitro-1-phenylethyl)-1H-indole (4ea): colorless solid, mp (EtOH) 139.9–142.1 °C, Rf 0.34 (EtOAc/Hex, 1:4). Yield: 640 mg (1.72 mmol, 86%). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (s, 1H), 7.52 (dd, J = 8.0, 1.0 Hz, 1H), 7.41–7.28 (m, 7H), 7.26–7.18 (m, 2H), 7.11 (ddd, J = 8.1, 7.1, 1.1 Hz, 1H), 7.03–6.96 (m, 2H), 5.32–5.27 (m, 1H), 5.22–5.08 (m, 2H), 3.87 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 160.00, 140.12, 137.03, 136.00, 130.2 (2C), 129.0 (2C), 127.6 (2C), 127.29, 127.20, 124.61, 122.36, 120.34, 119.89, 114.5 (2C), 111.40, 109.21, 79.24, 55.51, 40.99. IR, vmax/cm−1: 3402, 3041, 1607, 1546, 1501, 1460, 1441, 1371,1243, 1178. HRMS (ES TOF) calculated for (M + Na)+ C23H20N2NaO3 395.1366, found 395.1355 (2.9 ppm).
2-(4-Methoxyphenyl)-3-(1-(4-methoxyphenyl)-2-nitroethyl)-1H-indole (4ee): colorless solid, mp (EtOH) 145,6–146,9 °C Rf 0.15 (EtOAc/Hex, 1:4). Yield: 640 mg (1.6 mmol, 80%). 1H NMR (400 MHz, Chloroform-d) δ 8.12 (s, 1H), 7.54 (d, J = 7.9 Hz, 1H), 7.38 (m, J = 7.4 Hz, 3H), 7.28 (d, J = 3.2 Hz, 2H), 7.22 (m, J = 7.6 Hz, 1H), 7.13 (m, J = 7.6 Hz, 1H), 6.99 (d, J = 8.8 Hz, 2H), 6.84 (d, J = 8.8 Hz, 2H), 5.24 (m, J = 7.9 Hz, 1H), 5.19–5.07 (m, 2H), 3.88 (s, 3H), 3.79 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 160.0, 158.7, 136.9, 136.0, 132.2, 130.2 (2C), 128.7 (2C), 127.2, 124.7, 122.3, 120.3, 119.9, 114.5 (2C), 114.3 (2C), 111.4, 109.4, 79.5, 55.5, 55.4, 40.4. IR, vmax/cm−1: 3403, 1552, 1508, 1458, 1376, 1345, 1284, 1243, 1176. HRMS (ES TOF) calculated for (M + Na)+ C24H22N2NaO4 425.1463, found 425.1472 (2.1 ppm).
2-(4-Methoxy-3-methylphenyl)-3-(1-(2-methoxyphenyl)-2-nitroethyl)-1H-indole (4fd): yellow solid, mp (EtOH) 151.5–153.4 °C, Rf 0.50 (EtOAc/Hex, 1:4). Yield: 599 mg (1.44 mmol, 72%). 1H NMR (400 MHz, Chloroform-d) δ 8.09 (s, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.42–7.33 (m, 2H), 7.28–7.16 (m, 4H), 7.15–7.10 (m, 1H), 6.93–6.82 (m, 3H), 5.59 (t, J = 7.8 Hz, 1H), 5.14 (d, J = 7.8 Hz, 2H), 3.87 (s, 3H), 3.82 (s, 3H), 2.22 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 158.0, 156.8, 137.4, 136.0, 131.0, 129.4, 128.7, 128.0, 127.3, 127.1, 124.5, 122.0, 120.8, 120.2 (2C), 111.3, 110.6, 110.1, 108.4, 77.7, 77.4, 55.5, 55.4, 36.4, 16.5. IR, vmax/cm−1: 3408, 2834, 1552, 1385, 1243, 1026, 769. HRMS (ES TOF) calculated for (M + Na)+ C25H24N2NaO4 439.1628, found 439.1622 (1.5 ppm).
2-(4-Fluorophenyl)-3-(2-nitro-1-phenylethyl)-1H-indole (4ga): colorless powder, mp 185–187 °C, Rf 0.25 (EtOAc/Hex 1:8). Yield: 562 mg (1.56 mmol, 78 %). 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.60–7.51 (m, 2H), 7.46–7.35 (m, 3H), 7.35–7.25 (m, 4H), 7.25–7.17 (m, 1H), 7.16–7.04 (m, 1H), 6.98 (t, J = 7.6 Hz, 1H), 5.55 (dd, J = 13.2, 7.4 Hz, 1H), 5.44 (dd, J = 13.2, 9.1 Hz, 1H), 5.15 (t, J = 8.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.1 (d, J = 245.5 Hz), 140.2, 136.2, 135.7, 130.9 (d, J = 8.3 Hz, 2C), 128.9 (d, J = 3.1 Hz), 128.8 (2C), 127.35 (2C), 126.9, 126.2, 121.7, 120.0, 119.4, 115.9 (d, J = 21.6 Hz, 2C), 111.7, 108.8, 78.2, 40.3. IR, vmax/cm–1: 3417, 3060, 1901, 1655, 1544, 1452, 1375, 1214, 1161, 1026, 836. HRMS (ES TOF) calculated for (M + Na)+ C22H17FN2NaO2 383.1164, found 383.1166 (0.6 ppm).
2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-3-(2-nitro-1-phenylethyl)-1H-indole (4ha): colorless solid, mp (EtOH) 161.6–163.4 °C, Rf 0.25 (EtOAc/Hex, 1:4). Yield: 696 mg (1.74 mmol, 87%). 1H NMR (400 MHz, DMSO-d6) δ 11.36 (s, 1H), 7.63 (d, J = 7.9 Hz, 1H), 7.35 (d, J = 8.1 Hz, 1H), 7.32–7.26 (m, 4H), 7.20 (m, J = 8.5, 5.7, 2.2 Hz, 1H), 7.09 (m, J = 8.2, 7.0, 1.1 Hz, 1H), 7.05–7.00 (m, 3H), 6.96 (m, J = 8.1, 7.0, 1.1 Hz, 1H), 5.59–5.40 (m, 2H), 5.21 (t, J = 8.3 Hz, 1H), 4.31 (s, 4H). 13C NMR (101 MHz, DMSO-d6) δ 143.6, 143.5, 140.3, 136.2, 136.1, 128.7 (2C), 127.3 (2C), 126.8, 126.4, 125.5, 121.7, 121.4, 119.8, 119.2, 117.5, 117.2, 111.6, 108.0, 78.2, 64.23, 64.18, 40.3. IR, vmax/cm−1: 2993, 2680, 1773, 1718, 1653, 1508, 1458, 1361, 1243. HRMS (ES TOF) calculated for (M + Na)+ C24H20N2NaO4 423.1315, found 423.1302 (3.2 ppm).
2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-3-(1-(2-methoxyphenyl)-2-nitroethyl)-1H-indole (4hd): bright yellow solid, mp (EtOH) 182.1–183.7 °C, Rf 0.41 (EtOAc/Hex, 1:2). Yield: 636 mg (1.48 mmol, 74%). 1H NMR (400 MHz, Chloroform-d) δ 8.08 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.39–7.33 (m, 2H), 7.27–7.22 (m, 1H), 7.22–7.17 (m, 1H), 7.14–7.10 (m, 1H), 6.97 (d, J = 1.7 Hz, 1H), 6.92–6.89 (m, 3H), 6.85–6.83 (m, 1H), 5.60 (dd, J = 9.0, 6.5 Hz, 1H), 5.16 (d, J = 2.3 Hz, 1H), 5.14 (d, J = 4.7 Hz, 1H), 4.31–4.26 (m, 4H), 3.83 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 156.9, 144.0, 143.8, 136.8, 136.0, 129.3, 128.7, 127.9, 127.8, 125.9, 122.2, 122.0, 120.8, 120.24, 120.22, 117.8, 117.7, 111.4, 110.8, 108.8, 77.7, 64.6, 64.5, 55.5, 36.3. IR, vmax/cm−1: 3427, 1549, 1487, 1431, 1272, 747. HRMS (ES TOF) calculated for (M + Na)+ C25H22N2NaO5 453.1421, found 453.1410 (2.4 ppm).
5-Isopropyl-3-(2-nitro-1-phenylethyl)-2-phenyl-1H-indole (4ia): colorless solid, mp (EtOH) 165.9–166.9 °C, Rf 0.51 (EtOAc/Hex, 1:4). Yield: 602 mg (1.57 mmol, 78%). 1H NMR (400 MHz, Chloroform-d) δ 8.09 (s, 1H), 7.49–7.40 (m, 5H), 7.39–7.26 (m, 6H), 7.28–7.20 (m, 1H), 7.13 (dd, J = 8.3, 1.7 Hz, 1H), 5.32 (t, J = 7.8 Hz, 1H), 5.22 (dd, J = 12.5, 7.8 Hz, 1H), 5.10 (dd, J = 12.5, 7.9 Hz, 1H), 2.97 (h, J = 6.9 Hz, 1H), 1.28 (d, J = 6.9 Hz, 3H), 1.27 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 141.2, 140.2, 137.1, 134.7, 132.5, 129.1 (2C), 129.0 (2C), 128.9 (2C), 128.7, 127.7 (2C), 127.3, 127.3, 121.7, 117.2, 111.3, 109.7, 79.4, 40.9, 34.4, 24.9, 24.7. IR, vmax/cm−1: 3393, 2949, 1546, 1446, 1386, 1318, 1241. HRMS (ES TOF) calculated for (M + Na)+ C25H24N2NaO2 407.1730, found 407.1728 (0.4 ppm).
1-Methyl-3-(2-nitro-1-phenylethyl)-2-phenyl-1H-indole (
4ja): pale cream powder, mp (EtOH) 113–114 °C (Literature data: mp 98–99 °C [
34]), R
f 0.42 (EtOAc/Hex 1:8). Yield: 528 mg (1.48 mmol, 74%).
1H NMR (400 MHz, DMSO-
d6) δ 7.70 (d,
J = 8.0 Hz, 1H), 7.59 7.64–7.53 (m, 3H), 7.49 (d,
J = 8.2 Hz, 1H), 7.42 (d,
J = 7.1 Hz, 2H), 7.26 (d,
J = 5.5 Hz, 4H), 7.22–7.17 (m, 2H), 7.08–7.01 (m, 1H), 5.50 (dd,
J = 13.1, 7.6 Hz, 1H), 5.41 (dd,
J = 13.1, 9.0 Hz, 1H), 4.93–4.80 (m, 1H), 3.52 (s, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 140.2, 138.9, 136.9, 130.8, 130.6 (2C), 128.9, 128.7 (2C), 128.6 (2C), 127.2 (2C), 126.8, 125.1, 121.6, 119.8, 119.6, 110.3, 109.7, 77.9, 40.6, 30.7. IR, v
max/cm
–1: 3046, 1740, 1684, 1655, 1559, 1546, 1375, 1248, 1137, 1079, 920. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
20N
2NaO
2 379.1428, found 379.1417 (−3.0 ppm).
1-Methyl-3-(2-nitro-1-phenylethyl)-2-(p-tolyl)-1H-indole (4ka): colorless solid, mp (EtOH) 142.4–143.3 °C, Rf 0.69 (EtOAc/Hex, 1:4). Yield: 533 mg (1.44 mmol, 72%). 1H NMR (400 MHz, Chloroform-d) δ 7.54 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.29 (d, J = 7.9 Hz, 2H), 7.25 (m, J = 2.1 Hz, 5H), 7.23–7.16 (m, 3H), 7.12 (m, J = 8.1, 7.1, 1.2 Hz, 1H), 5.09 (d, J = 9.2 Hz, 2H), 5.05–5.01 (m, 1H), 3.52 (s, 3H), 2.45 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 140.3, 139.9, 138.9, 137.3, 130.7 (2C), 129.4 (2C), 128.8 (2C), 128.2, 127.5 (2C), 127.1, 126.0, 121.9, 119.9, 119.7, 110.0, 109.8, 79.1, 41.3, 30.9, 21.6. IR, vmax/cm−1: 3032, 2974, 1817, 1552, 1366, 1250, 1185, 1140, 1017. HRMS (ES TOF) calculated for (M + Na)+ C24H22N2NaO2 393.1573, found 393.1561 (3.2 ppm).
7-Chloro-3-(2-nitro-1-phenylethyl)-2-phenyl-1H-indole (4la): colorless solid, mp (EtOH) 122.3–124.1 °C, Rf 0.57 (EtOAc/Hex, 1:4). Yield: 430 mg (1.14 mmol, 57%). 1H NMR (400 MHz, Chloroform-d) δ 8.38 (s, 1H), 7.49 (d, J = 3.1 Hz, 5H), 7.44–7.39 (m, 1H), 7.34–7.25 (m, 5H), 7.23 (dd, J = 7.7, 0.8 Hz, 1H), 7.06 (t, J = 7.9 Hz, 1H), 5.31 (dd, J = 8.9, 7.1 Hz, 1H), 5.22–5.08 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 139.6, 137.9, 133.4, 131.7, 129.20 (2C), 129.17 (2C), 129.1, 129.0 (2C), 128.4, 127.50 (2C), 127.47, 122.0, 121.3, 118.6, 117.1, 110.7, 79.0, 40.8. IR, vmax/cm−1: 3402, 3046, 1542, 1492, 1443, 1378, 1311, 1248. HRMS (ES TOF) calculated for (M + Na)+ C22H17ClN2NaO2 399.0871, found 399.0862 (2.2 ppm).
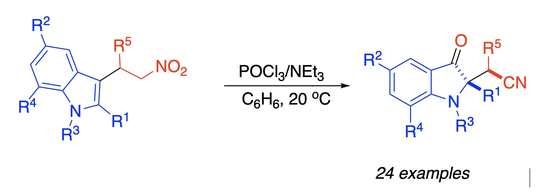
Preparation of 2-(1H-indol-2-yl)acetonitriles 6 (general procedure): A 10 mL round bottom flask was charged with indolylnitroethane 4 (1.0 mmol), POCl3 (2.0 mmol, 306 mg, 187 μL), and benzene (5 mL). The mixture was stirred at room temperature for 5 min, and then Et3N (4.0 mmol, 404 mg, 557 μL) was added in one portion. The mixture was stirred for another 30 min, and the reaction progress was monitored by TLC. After complete consumption of the starting material, the mixture was diluted with water (30 mL), extracted with EtOAc (4 × 15 mL), and the combined organic extracts were concentrated in vacuo. The residue was purified by preparative column chromatography eluting with EtOAc/Hex 1:4, to afford pure nitrile 6.
2-(2-Methoxyphenyl)-2-(2-methyl-3-oxoindolin-2-yl)acetonitrile (6ad): yellow solid, mp (EtOH) 145.6–148.9 °C, Rf 0.36 (EtOAc/Hex, 1:2). Yield: 155 mg (0.53 mmol, 53%). 1H NMR (400 MHz, Chloroform-d) δ 7.68 (d, J = 7.8 Hz, 1H), 7.60–7.54 (m, 1H), 7.52–7.46 (m, 1H), 7.41–7.32 (m, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.98–6.86 (m, 3H), 4.76 (s, 1H), 4.69 (s, 1H), 3.88 (s, 3H), 1.17 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 201.5, 160.2, 157.0, 137.9, 130.5, 130.4, 125.5, 121.4, 120.4, 120.2, 120.1, 118.4, 113.0, 111.2, 68.3, 55.8, 37.1, 20.9. IR, vmax/cm−1: 3311, 2839, 2231, 1687, 1487, 1323, 1265, 1034. HRMS (ES TOF) calculated for (M + Na)+ C18H16N2NaO2 315.1104, found 315.1097 (2.3 ppm).
2-(3-Oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6ba): yellow solid, mp (EtOH) 197.1–198.2 °C (Literature data: mp 188.4–190.3 °C [
20]), R
f 0.15 (Benzene). Yield: 233 mg (0.72 mmol, 72%).
1H NMR (400 MHz, DMSO-
d6) δ 8.16 (s, 1H), 7.58–7.52 (m, 3H), 7.46 (d,
J = 7.7 Hz, 1H), 7.30 (m,
J = 6.5 Hz, 1H), 7.25 (m,
J = 7.0, 3.6 Hz, 7H), 7.13 (d,
J = 8.3 Hz, 1H), 6.78 (t,
J = 7.4 Hz, 1H), 5.29 (s, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 198.8, 161.8, 138.2, 134.7, 131.6, 129.4 (2C), 128.42 (2C), 128.36 (2C), 128.28, 126.3 (2C), 124.8, 119.0, 118.7, 117.7, 112.5, 73.2, 43.4. IR, v
max/cm
−1: 3359, 2940, 2241, 1699, 1508, 1489, 1323, 1243, 1053. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
16N
2NaO 347.1155, found 347.1148 (1.8 ppm).
2-(3-Oxo-2-phenylindolin-2-yl)-2-(p-tolyl)acetonitrile (6bb): yellow solid, mp (EtOH) 178.7–183.9 °C, Rf 0.33 (EtOAc/Hex, 1:4). Yield: 257 mg (0.76 mmol, 76%). 1H NMR (400 MHz, DMSO-d6) δ 8.12 (s, 1H), 7.59–7.50 (m, 3H), 7.45 (d, J = 7.7 Hz, 1H), 7.33–7.21 (m, 3H), 7.13–7.03 (m, 5H), 6.78 (t, J = 7.4 Hz, 1H), 5.24 (s, 1H), 2.21 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.8, 161.8, 138.2, 137.7, 134.8, 129.3 (2C), 128.9 (2C), 128.6, 128.4 (2C), 128.2, 126.3 (2C), 124.7, 119.1, 118.6, 117.6, 112.5, 73.3, 43.0, 20.6. IR, vmax/cm−1: 3335, 2246, 1677, 1619, 1494, 1465, 1325, 1296, 1239. HRMS (ES TOF) calculated for (M + Na)+ C23H18N2NaO 361.1311, found 361.1315 (−1.0 ppm).
2-(4-Isopropylphenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (6bc): yellow solid, mp (EtOH) 177.2–178.6 °C, Rf 0.37 (EtOAc/Hex, 1:4). Yield: 205 mg (0.56 mmol, 56%). 1H NMR (400 MHz, DMSO-d6) δ 8.13 (s, 1H), 7.59–7.52 (m, 3H), 7.44 (d, J = 6.8 Hz, 1H), 7.32–7.27 (m, 3H), 7.28–7.24 (m, 1H), 7.14 (m, 4H), 6.78 (t, J = 6.9 Hz, 1H), 5.27 (s, 1H), 2.80 (p, J = 7.0 Hz, 1H), 1.12 (dd, J = 7.0, 5.1 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 198.9, 161.9, 148.4, 138.2, 134.7, 129.4 (2C), 129.0, 128.4 (2C), 128.3, 126.34 (2C), 126.30 (2C), 124.7, 119.1, 118.6, 117.6, 112.6, 73.3, 42.9, 33.0, 23.8, 23.6. IR, vmax/cm−1: 3056, 2974, 2366, 1737, 1720, 1653, 1559, 1540, 1491, 1243, 1058. HRMS (ES TOF) calculated for (M + Na)+ C25H22N2NaO 389.1624, found 389.1611 (3.5 ppm).
2-(2-Methoxyphenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (6bd): yellow solid, mp (EtOH) 186.9–188.2 °C, Rf 0.36 (EtOAc/Hex, 1:2). Yield: 110 mg (0.34 mmol, 62%). 1H NMR (400 MHz, Chloroform-d) δ 7.64 (d, J = 7.8 Hz, 1H), 7.51–7.43 (m, 3H), 7.42–7.38 (m, 1H), 7.25–7.17 (m, 4H), 6.98 (d, J = 8.2 Hz, 1H), 6.94–6.82 (m, 2H), 6.69 (d, J = 8.3 Hz, 1H), 5.65 (s, 1H), 5.15 (s, 1H), 3.53 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 198.9, 160.4, 156.6, 138.1, 135.00, 130.7, 130.5, 128.4, 128.0 (2C), 126.3 (2C), 125.7, 121.1, 120.2, 120.1, 120.0, 118.0, 112.5, 111.2, 72.2, 55.5, 39.7. IR, vmax/cm−1: 3374, 2843, 2236, 1681, 1487, 1325, 1251, 1029. HRMS (ES TOF) calculated for (M + Na)+ C23H18N2NaO2 377.1260, found 377.1251 (2.6 ppm).
2-(4-Methoxyphenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (
6be): yellow solid, mp (EtOH) 187.2–188.5 °C (Literature data: mp 175.4–176.2 °C [
20]), R
f 0.34 (EtOAc/Hex, 1:4). Yield: 199 mg (0.56 mmol, 56%).
1H NMR (400 MHz, Chloroform-
d) δ 7.62 (d,
J = 7.8 Hz, 1H), 7.54–7.46 (m, 1H), 7.47–7.41 (m, 2H), 7.33–7.26 (m, 3H), 7.02–6.97 (m, 1H), 6.98–6.93 (m, 2H), 6.89–6.83 (m, 1H), 6.74–6.68 (m, 2H), 5.18 (s, 1H), 4.66 (s, 1H), 3.74 (s, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 198.5, 160.1, 159.9, 138.2, 134.9, 130.2 (2C), 128.8 (2C), 128.8, 126.0 (2C), 125.8, 122.6, 120.3, 119.8, 118.1, 114.1 (2C), 112.3, 72.5, 55.4, 45.1.
1H NMR (400 MHz, DMSO-
d6) δ 8.13 (s, 1H), 7.59–7.51 (m, 3H), 7.45 (d,
J = 7.7 Hz, 1H), 7.35–7.21 (m, 3H), 7.18–7.10 (m, 3H), 6.84–6.75 (m, 3H), 5.22 (s, 1H), 3.68 (s, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 198.9, 161.8, 159.1, 138.2, 134.9, 130.6 (2C), 128.4 (2C), 128.2, 126.3 (2C), 124.7, 123.4, 119.2, 118.6, 117.7, 113.7 (2C), 112.5, 73.4, 55.1, 42.6. IR, v
max/cm
−1: 3326, 2250, 1672, 1617, 1510, 1487, 1462, 1325, 1257. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
18N
2NaO
2 377.1260, found 377.1250 (2.7 ppm).
2-(2-Fluorophenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (
6bf): yellow solid, mp (EtOH) 181.9–184.4 °C (Literature data: mp 176.4–179.0 °C [
20]), R
f 0.28 (benzene). Yield: 219 mg (0.64 mmol, 64%).
1H NMR (400 MHz, DMSO
d6) δ 8.34 (s, 1H), 7.60–7.46 (m, 5H), 7.38–7.30 (m, 1H), 7.30–7.19 (m, 4H), 7.13 (d,
J = 8.3 Hz, 1H), 7.07 (ddd,
J = 9.7, 8.3, 1.2 Hz, 1H), 6.80 (ddd,
J = 7.8, 7.0, 0.8 Hz, 1H), 5.23 (s, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 198.6, 161.9, 159.73 (d,
J = 248.6 Hz), 138.6, 134.5, 131.3, 131.21, 131.21 (d,
J = 5.3 Hz), 128.7, 128.5 (2C), 126.2 (2C), 125.0, 124.96 (d,
J = 3.6 Hz), 119.0, 118.81 (d,
J = 13.9 Hz), 117.95 (d,
J = 22.3 Hz), 115.89 (d,
J = 22.0 Hz), 112.8, 72.7, 38.0. IR, v
max/cm
−1: 3369, 2246, 1663, 1610, 1496, 1460, 1325, 1246, 1159. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
15FN
2NaO 365.1061, found 365.1067 (−1.9 ppm).
2-(4-Fluorophenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (
6bg): yellow solid, mp (EtOH) 170.7–173.3 °C (Literature data: mp 172.5–174.1 °C [
20]), R
f 0.23 (EtOAc/Hex, 1:4). Yield: 270 mg (0.79 mmol, 79%).
1H NMR (400 MHz, DMSO
d6) δ 8.16 (s, 1H), 7.60–7.51 (m, 3H), 7.46 (d,
J = 7.7 Hz, 1H), 7.35–7.20 (m, 5H), 7.16–7.07 (m, 3H), 6.79 (t,
J = 7.5 Hz, 1H), 5.35 (s, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 198.8, 161.8, 162.0 (d,
J = 245.5 Hz), 138.3, 134.6, 131.5 (d,
J = 8.2 Hz, 2C), 128.5 (2C), 128.4, 127.9 (d,
J = 2.8 Hz), 126.3 (2C), 124.7, 118.9, 118.8, 117.7, 115.3 (d,
J = 21.8 Hz, 2C), 112.6, 73.2, 42.6. IR, v
max/cm
−1: 3350, 3056, 2255, 1684, 1619, 1518, 1491, 1463, 1318, 1221, 1149. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
15FN
2NaO 365.1061, found 365.1050 (2.9 ppm).
2-(4-Chlorophenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (
6bh): yellow solid, mp (EtOH) 189.5–191.1 °C (Literature data: mp 213–214 °C [
20]), R
f 0.26 (EtOAc/Hex, 1:4). Yield: 226 mg (0.63 mmol, 63%).
1H NMR (400 MHz, DMSO-
d6) δ 8.15 (s, 1H), 7.59–7.51 (m, 3H), 7.46 (d,
J = 7.0 Hz, 1H), 7.36 (d,
J = 2.0 Hz, 1H), 7.34 (q,
J = 2.1 Hz, 2H), 7.31–7.26 (m, 2H), 7.24–7.19 (m, 2H), 7.11 (d,
J = 8.3 Hz, 1H), 6.79 (t,
J = 7.4 Hz, 1H), 5.38 (s, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 198.7, 161.7, 138.3, 134.6, 133.3, 131.2 (2C), 130.6, 128.6 (2C), 128.4 (3C), 126.3 (2C), 124.7, 118.8, 118.7, 117.7, 112.5, 73.1, 42.8. IR, v
max/cm
−1: 3359, 2362, 1737, 1653, 1545, 1508, 1489, 1455, 1238. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
15ClN
2NaO 381.0765, found 381.0758 (1.9 ppm).
2-(4-(Dimethylamino)phenyl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (6bi): yellow solid, mp (EtOH) 235.7–237.7 °C, Rf 0.34 (EtOAc/Hex, 1:3). Yield: 187 mg (0.51 mmol, 51%). 1H NMR (400 MHz, DMSO-d6) δ 8.09 (s, 1H), 7.59–7.49 (m, 3H), 7.43 (d, J = 7.8 Hz, 1H), 7.33–7.18 (m, 3H), 7.12 (d, J = 8.3 Hz, 1H), 7.06–6.98 (m, 2H), 6.77 (t, J = 7.4 Hz, 1H), 6.60–6.52 (m, 2H), 5.09 (s, 1H), 2.82 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 199.0, 161.8, 150.0, 138.0, 135.1, 130.0 (2C), 128.4 (2C), 128.1, 126.3 (2C), 124.6, 119.4, 118.5, 118.2, 117.7, 112.5, 111.9 (2C), 73.6, 42.6, 39.9 (2C). IR, vmax/cm−1: 2979, 2366, 1829, 1706, 1655, 1545, 1508, 1492, 1238. HRMS (ES TOF) calculated for (M + Na)+ C24H21N3NaO 390.1577, found 390.1568 (2.2 ppm).
2-(3,5-Dimethyl-1H-pyrazol-4-yl)-2-(3-oxo-2-phenylindolin-2-yl)acetonitrile (6bj): yellow solid, mp (EtOH) 184.9–188.2 °C, Rf 0.11 (EtOAc/Hex, 1:1). Yield: 89 mg (0.26 mmol, 26%). 1H NMR (400 MHz, Acetone-d6) δ 11.51 (s, 1H), 7.70–7.62 (m, 2H), 7.59–7.49 (m, 2H), 7.36–7.26 (m, 4H), 7.18 (dd, J = 8.2, 1.0 Hz, 1H), 6.89–6.78 (m, 1H), 4.65 (s, 1H), 1.93 (s, 6H). 13C NMR (101 MHz, Acetone-d6) δ 199.7, 162.1, 144.2 (2C), 138.7, 136.7, 129.3, 129.2 (2C), 127.5 (2C), 125.5, 120.3, 119.8, 118.7, 113.2, 105.4, 74.6, 37.0, 11.4 (2C). IR, vmax/cm−1: 3321, 2241, 1675, 1624, 1496, 1306, 1251, 1202, 1157. HRMS (ES TOF) calculated for (M + Na)+ C21H18N4NaO 365.1373, found 365.1373 (1.9 ppm).
2-(3-Oxo-2-phenylindolin-2-yl)-2-(p-tolyl)acetonitrile (
6ca): yellow solid, mp (EtOH) 181.6–182.9 °C [
20]), R
f 0.29 (EtOAc/Hex, 1:4). Yield: 274 mg (0.81 mmol, 81%).
1H NMR (400 MHz, DMSO-
d6) δ 8.10 (s, 1H), 7.53 (t,
J = 7.7 Hz, 1H), 7.44 (dd,
J = 7.8, 5.3 Hz, 3H), 7.26 (s, 5H), 7.10 (dd,
J = 11.7, 8.2 Hz, 3H), 6.77 (t,
J = 7.4 Hz, 1H), 5.25 (s, 1H), 2.21 (s, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 198.9, 161.8, 138.1, 137.6, 131.7 (2C), 129.4 (2C), 129.0 (2C), 128.4 (2C), 128.3, 126.2 (2C), 124.6, 119.0, 118.6, 117.7, 112.5, 73.0, 43.3, 20.5. IR, v
max/cm
−1: 3364, 3094, 2251, 1680, 1629, 1513, 1484, 1472, 1325, 1234. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
18N
2NaO
2 1.1, found 1.1 (2.0 ppm).
2-(2-(3,5-Dimethylphenyl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (6da): yellow solid, Rf 0.46 (EtOAc/Hex, 1:2). Yield: 145 mg (0.41 mmol, 41%). 1H NMR (400 MHz, DMSO-d6) δ 8.05 (s, 1H), 7.53 (t, J = 7.7 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.34 (s, 1H), 7.26 (s, 6H), 7.11 (d, J = 8.3 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.77 (t, J = 7.4 Hz, 1H), 5.25 (s, 1H), 2.13 (d, J = 9.9 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 199.0, 161.8, 138.1, 136.3, 136.2, 132.0, 131.8, 129.5 (3C), 128.4 (2C), 128.3, 127.2, 124.6, 123.7, 119.1, 118.6, 117.7, 112.6, 73.1, 43.2, 19.6, 19.0. IR, vmax/cm−1: 3350, 2246, 1687, 1617, 1494, 1470, 1330, 1303, 1101, 1069. HRMS (ES TOF) calculated for (M + Na)+ C24H20N2NaO 375.1478, found 375.1468 (−2.8 ppm).
2-(2-(4-Methoxyphenyl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6ea): yellow solid, mp (EtOH) 174.9–178.3 °C (Literature data: mp 170.7–172.8 °C [
20]), R
f 0.17 (EtOAc/Hex, 1:4). Yield: 216 mg (0.61 mmol, 61%).
1H NMR (400 MHz, Chloroform-
d) δ 7.64 (d,
J = 7.7 Hz, 1H), 7.50 (ddd,
J = 8.4, 7.1, 1.4 Hz, 1H), 7.36–7.19 (m, 5H), 7.06 (d,
J = 7.1 Hz, 2H), 6.98 (d,
J = 8.3 Hz, 1H), 6.88 (t,
J = 7.4 Hz, 1H), 6.84–6.73 (m, 2H), 5.06 (s, 1H), 4.65 (s, 1H), 3.76 (s, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 198.7, 160.0, 159.9, 138.2, 130.9, 129.1 (2C), 128.9, 128.8 (2C), 127.3 (2C), 126.5, 125.9, 120.3, 119.9, 117.9, 114.2 (2C), 112.3, 72.0, 55.4, 45.7. IR, v
max/cm
−1: 3311, 2968, 2231, 1681, 1609, 1513, 1494, 1472, 1330, 1257, 1190, 1152. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
18N
2NaO
2 377.1260, found 377.1250 (2.7 ppm).
2-(4-Methoxyphenyl)-2-(2-(4-methoxyphenyl)-3-oxoindolin-2-yl)acetonitrile (6ee): yellow oil, Rf 0.29 (EtOAc/Hex, 1:2). Yield: 227 mg (0.59 mmol, 59%). 1H NMR (400 MHz, DMSO-d6) δ 8.04 (s, 1H), 7.52 (t, J = 7.7 Hz, 1H), 7.44 (m, J = 7.3 Hz, 3H), 7.14 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.3 Hz, 1H), 6.84 (dd, J = 8.9, 4.8 Hz, 4H), 6.77 (t, J = 7.3 Hz, 1H), 5.15 (s, 1H), 3.69 (d, J = 2.8 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 199.2, 161.7, 159.09, 159.05, 138.0, 130.6 (2C), 127.6 (2C), 126.5, 124.6, 123.5, 119.2, 118.5, 117.8, 113.77 (2C), 113.75 (2C), 112.5, 72.9, 55.11 (2C), 42.6. IR, vmax/cm−1: 2926, 2858, 2241, 1701, 1610, 1511, 1465, 1248, 1183, 1026. HRMS (ES TOF) calculated for (M + Na)+ C24H20N2NaO3 407.1354, found 407.1366 (3.0 ppm).
2-(2-(4-Methoxy-3-methylphenyl)-3-oxoindolin-2-yl)-2-(2-methoxyphenyl)acetonitrile (6fd): pale yellow solid, mp (EtOH) 138.9–141.8 °C, Rf 0.39 (EtOAc/Hex, 1:2). Yield: 247 mg (0.62 mmol, 62%). 1H NMR (400 MHz, DMSO-d6) δ 8.27 (s, 1H), 7.59–7.52 (m, 2H), 7.48 (d, J = 7.1 Hz, 1H), 7.31–7.24 (m, 1H), 7.22–7.19 (m, 1H), 7.16 (d, J = 8.2 Hz, 1H), 7.13–7.10 (m, 1H), 7.02–6.975 (m, 1H), 6.87 (d, J = 8.4 Hz, 1H), 6.79 (t, J = 7.5 Hz, 1H), 6.73 (d, J = 8.7 Hz, 1H), 5.22 (s, 1H), 3.66 (s, 3H), 3.58 (s, 3H), 2.03 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 199.5, 162.3, 157.5, 156.6, 138.6, 130.6, 129.9, 128.6, 125.8, 125.3, 125.1, 121.1, 120.2, 119.2, 119.1, 118.2, 113.1, 111.7, 109.9, 72.9, 56.5, 55.9, 55.7, 36.5, 16.7. IR, vmax/cm−1: 3282, 2844, 2251, 1687, 1489, 1320, 1248, 1026. HRMS (ES TOF) calculated for (M + Na)+ C25H22N2NaO3 421.1523, found 421.1511 (2.8 ppm).
2-(2-(4-Fluorophenyl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (6ga): light yellow crystals, mp 191.7–193.1 °C, Rf 0.33 (EtOAc/Hex 1:4). Yield: 209 mg (0.61 mmol, 61%). 1H NMR (400 MHz, Chloroform-d) δ 7.68 (d, J = 7.8 Hz, 1H), 7.60–7.51 (m, 1H), 7.51–7.41 (m, 2H), 7.37–7.23 (m, 3H), 7.10–6.96 (m, 5H), 6.93 (t, J = 7.4 Hz, 1H), 5.19 (s, 1H), 4.68 (s, 1H). 13C NMR (101 MHz, Chloroform-d) δ 198.4, 162.9 (d, J = 248.8 Hz), 159.9, 138.4, 130.6 (d, J = 3.2 Hz), 130.6, 129.1, 129.0 (2C), 128.9 (2C), 128.0 (d, J = 8.3 Hz, 2C), 125.9, 120.5, 119.6, 117.6, 115.8 (d, J = 21.6 Hz, 2C), 112.3, 71.7, 45.9. IR, vmax/cm–1: 3311, 3065, 2251, 1689, 1624, 1590, 1508, 1467, 1328, 1301, 1224, 1161, 1010, 899. HRMS (ES TOF) calculated for (M + Na)+ C22H15FN2NaO 365.1050, found 365.1061 (3.0 ppm).
2-(2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6ha): yellow solid, mp (EtOH) 191–192.7 °C (Literature data: mp 99–101 °C [
20]), R
f 0.18 (EtOAc/Hex, 1:3). Yield: 290 mg (0.76 mmol, 76%).
1H NMR (400 MHz, DMSO
d6) δ 7.55 (s, 1H), 7.06 (t,
J = 8.4 Hz, 1H), 6.98 (d,
J = 7.8 Hz, 1H), 6.85–6.75 (m, 5H), 6.63 (d,
J = 8.3 Hz, 1H), 6.60–6.52 (m, 2H), 6.31 (t,
J = 7.6 Hz, 2H), 4.75 (s, 1H), 3.69 (s, 4H).
13C NMR (101 MHz, DMSO-
d6) δ 198.9, 161.7, 143.3, 143.07, 138.1, 131.7, 129.4 (2C), 128.39 (2C), 128.35, 127.5, 124.7, 119.3, 119.0, 118.6, 117.7, 116.9, 115.26, 112.49, 72.7, 64.1, 64.0, 43.2. IR, v
max/cm
−1: 2988, 2357, 1745, 1699, 1684, 1619, 1545, 1511, 1378, 1241. HRMS (ES TOF) calculated for (M + Na)
+ C
24H
18N
2NaO
3 405.1210, found 405.12 (2.5 ppm).
2-(2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-3-oxoindolin-2-yl)-2-(2-methoxyphenyl)acetonitrile (6hd): pale yellow solid, mp (EtOH) 168.4–171.5 °C, Rf 0.45 (EtOAc/Hex, 1:1). Yield: 239 mg (0.58 mmol, 58%). 1H NMR (400 MHz, Chloroform-d) δ 7.62 (d, J = 7.8 Hz, 1H), 7.50–7.42 (m, 1H), 7.41–7.37 (m, 1H), 7.26–7.20 (m, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.95 (d, J = 8.2 Hz, 1H), 6.92–6.87 (m, 2H), 6.84 (t, J = 7.4 Hz, 1H), 6.77–6.72 (m, 1H), 6.66 (d, J = 8.6 Hz, 1H), 5.57 (s, 1H), 5.10 (s, 1H), 4.15 (s, 4H), 3.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 198.8, 160.3, 156.7, 143.6, 143.1, 138.0, 130.7, 130.4, 128.1, 125.7, 121.1, 120.13, 120.10, 120.0, 119.5, 118.1, 116.8, 115.7, 112.4, 111.3, 71.9, 64.5, 64.3, 55.6, 39.3. IR, vmax/cm−1: 3417, 2845, 2246, 1682, 1492, 1255, 1036. HRMS (ES TOF) calculated for (M + Na)+ C25H20N2NaO4 435.1315, found 435.1306 (2.5 ppm).
2-(5-Isopropyl-3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6ia): yellow solid, mp (EtOH) 159.7–161.0 °C (Literature data: mp 150.7–152.6 °C [
20]), R
f 0.4 (EtOAc/Hex, 1:4). Yield: 287 mg (0.78 mmol, 78%).
1H NMR (400 MHz, DMSO-
d6) δ 7.98 (s, 1H), 7.59–7.52 (m, 2H), 7.49 (dd,
J = 8.5, 1.9 Hz, 1H), 7.26 (td,
J = 8.6, 6.8, 3.3 Hz, 9H), 7.09 (d,
J = 8.5 Hz, 1H), 5.29 (s, 1H), 2.95–2.66 (m, 1H), 1.15 (d,
J = 6.8 Hz, 3H), 1.15 (d,
J = 6.9 Hz, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 198.8, 160.6, 139.0, 137.5, 134.9, 131.8, 129.42 (2C), 128.39 (2C), 128.36 (2C), 128.3, 128.2, 126.2 (2C), 121.0, 119.1, 117.6, 112.7, 73.7, 43.3, 32.6, 23.9, 23.8. IR, v
max/cm
−1: 3396, 2964, 2236, 1701, 1619, 1491, 1453, 1349, 1272, 1241, 1171. HRMS (ES TOF) calculated for (M + Na)
+ C
25H
22N
2NaO 389.1624, found 389.1615 (2.5 ppm).
2-(1-Methyl-3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6ja): lemon-yellow crystals, mp 196–198 °C (Literature data: mp 174.8−178.3 °C [
20]), R
f 0.27 (EtOAc/Hex 1:4). Yield: 95 mg (0.28 mmol, 28%).
1H NMR (400 MHz, Chloroform-
d) δ 7.41 (d,
J = 7.7 Hz, 1H), 7.33–7.22 (m, 5H), 7.22–7.12 (m, 3H), 7.06 (h,
J = 4.6, 3.8 Hz, 3H), 6.57 (t,
J = 7.4 Hz, 1H), 6.48 (d,
J = 8.3 Hz, 1H), 4.77 (s, 1H), 2.63 (s, 3H).
13C NMR (101 MHz, Chloroform-
d) δ 198.7, 161.2, 138.3, 134.7, 130.5, 129.4 (2C), 129.3 (2C), 129.2, 129.1, 128.9, 128.5, 128.4, 127.2, 127.2, 125.3, 119.7, 118.3, 108.1, 77.5, 41.3, 30.5. IR, v
max/cm
–1: 3046, 2921, 2241, 1696, 1617, 1491, 1458, 1366, 1320, 1253, 1158, 1072, 1007, 971, 754. HRMS (ES TOF) calculated for (M + Na)
+ C
23H
18N
2NaO 361.1312, found 361.1311 (−0.3 ppm).
2-(1-Methyl-3-oxo-2-(p-tolyl)indolin-2-yl)-2-phenylacetonitrile (6ka): yellow solid, mp (EtOH) 168.7–170.1 °C, Rf 0.28 (EtOAc/Hex, 1:4). Yield: 81 mg (0.23 mmol, 23%). 1H NMR (400 MHz, DMSO-d6) δ 7.39–7.35 (m, 1H), 7.35–7.32 (m, 1H), 7.28–7.24 (m, 5H), 7.24–7.15 (m, 4H), 6.69 (d, J = 8.3 Hz, 1H), 6.59 (t, J = 7.3 Hz, 1H), 5.75 (s, 1H), 2.75 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 199.5, 160.6, 138.5, 138.3, 132.6, 131.0, 129.5 (2C), 129.1 (2C), 128.4, 128.1 (2C), 127.3 (2C), 123.7, 119.6, 118.6, 117.5, 108.8, 74.9, 38.2, 28.7, 20.7. IR, vmax/cm−1: 3036, 2926, 2236, 1701, 1614, 1489, 1371, 1323, 1161, 1024. HRMS (ES TOF) calculated for (M + Na)+ C24H20N2NaO 375.1468, found 375.1456 (3.3 ppm).
2-(7-Chloro-3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6la): yellow solid, mp (EtOH) 195.1–196.9 °C (Literature data: mp 188.7–189.6 °C [
20]), R
f 0.54 (EtOAc/Hex, 1:4). Yield: 127 mg (0.49 mmol, 49%).
1H NMR (400 MHz, DMSO-
d6) δ 8.35 (s, 1H), 7.81–7.71 (m, 2H), 7.57 (dd,
J = 7.6, 1.1 Hz, 1H), 7.45–7.29 (m, 6H), 7.28–7.18 (m, 3H), 6.71 (t,
J = 7.7 Hz, 1H), 5.22 (s, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 198.3, 156.9, 137.3, 134.8, 130.7, 129.6 (2C), 128.5 (2C), 128.5, 128.5, 128.2 (2C), 126.8 (2C), 123.2, 120.3, 119.3, 118.5, 116.0, 72.6, 43.7. IR, v
max/cm
−1: 3272, 3050, 2251, 1691, 1604, 1491, 1436, 1316, 1260, 1192, 1125. HRMS (ES TOF) calculated for (M + Na)
+ C
22H
15ClN
2NaO 381.0765, found 381.0754 (3.0 ppm).
Isolation of intermediate spirane 5: A 10 mL round bottom flask was charged with indolylnitroethane 4ba (342 mg, 1.00 mmol) and POCl3 (306 mg, 187 μL, 2.00 mmol). The resulting mixture was stirred at room temperature for 5 min. Next, the mixture was cooled in ice bath and triethylamine (404 mg, 557 μL, 4.00 mmol,) was added over a period of 5 min while maintaining the temperature below 10 °C. The reaction mixture was stirred for an additional 5 min, then diluted with water (30 mL), extracted with EtOAc (4 × 15 mL), and concentrated in vacuo. The residue was purified by column chromatography (eluent EtOAc/Hex 1:4), to afford three fractions: unreacted 3-(2-nitro-1-phenylethyl)-2-phenyl-1H-indole (4ba) (90 mg, 0.26 mmol, 26%), 2-(3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (6ba) (80 mg, 0.25 mmol, 25%), and 2,4′-diphenyl-4′H-spiro[indole-3,5′-isoxazole] (5) (40 mg, 0.13 mmol, 13%).
2,4′-Diphenyl-4′H-spiro[indole-3,5′-isoxazole] (5): yellow solid, Rf 0.4 (EtOAc/Hex, 1:4), Rf 0.49 (benzene). Yield: 40 mg (0.13 mmol, 13%). 1H NMR (400 MHz, Chloroform-d) δ 8.21–8.13 (m, 2H), 7.71 (t, J = 1.6 Hz, 1H), 7.61–7.49 (m, 3H), 7.40 (d, J = 7.7 Hz, 1H), 7.18–7.07 (m, 4H), 6.97–6.81 (m, 4H), 5.10 (s, 1H). 13C NMR (101 MHz, Chloroform-d) δ 177.3, 152.9, 149.5, 135.7, 133.1, 131.8, 131.7, 130.1, 129.2 (2C), 128.7 (2C), 128.3 (2C), 128.2, 127.8 (2C), 126.0, 124.7, 121.2, 97.7, 61.7. IR, vmax/cm−1: 3378, 3036, 2988, 1749, 1699, 1653, 1559, 1525, 1505, 1489, 1458, 1376, 1243, 1051. HRMS (ES TOF) calculated for (M + H)+ C22H17N2O 325.1335, found 325.1330 (1.6 ppm).