Scanning Electron Microscopy Investigation for Monitoring the Emulsion Deteriorative Process and Its Applications in Site-Directed Reaction with Paper Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
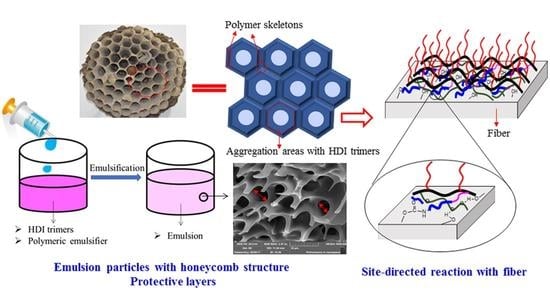
2.2. Preparation of Micrometer Emulsion
2.3. Characterization of Deteriorative Processes for Emulsion
2.3.1. -NCO Content Measurement
2.3.2. SEM Investigation
2.3.3. FT-IR Spectroscopy
2.4. Characterization of Sizing-Treated Paper Fabric
2.4.1. Dynamic Contact Angles
2.4.2. Surface Free Energy
3. Results and Discussion
3.1. Microstructure of Emulsion
3.2. Effect of Time on Deteriorative Processes of Emulsion
3.2.1. -NCO Content and SEM Investigation
3.2.2. FT-IR Spectroscopy
3.3. Effect of Temperature on Deteriorative Processes of Emulsion
3.3.1. -NCO Content and SEM Investigation
3.3.2. FT-IR Spectroscopy
3.4. The Site-Directed Reaction of Active Substances with Paper Fibers
3.4.1. Dynamic Contact Angles
3.4.2. Surface Free Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Decostanzi, M.; Auvergne, R.; Darroman, E.; Boutevin, B.; Caillol, S. Reactivity and kinetics of HDI-iminooxadiazinedione: Application to polyurethane synthesis. Eur. Polym. J. 2017, 96, 443–451. [Google Scholar] [CrossRef]
- Libni, G.; Nasar, A.S. Catalysis of Forward and Reverse Reactions of epsilon-Caprolactam-Blocked Polyisocyanate: Double Arrhenius Plots and Equilibrium Temperatures of a Thermally Reversible Reaction. ChemistrySelect 2017, 2, 9586–9594. [Google Scholar] [CrossRef]
- Poljanšek, I.; Fabjan, E.; Moderc, D.; Kukanja, D. The effect of free isocyanate content on properties of one component urethane adhesive. Int. J. Adhes. 2014, 51, 87–94. [Google Scholar] [CrossRef]
- Li, A.; Fan, G.; Chen, H.; Zhao, Q. Synthesis and characterization of water-borne diisocyanate crosslinkers from methyl ethyl ketoxime/2-methylimidazole-blocked aromatic isocyanates. Res. Chem. Intermediat. 2013, 39, 3565–3577. [Google Scholar] [CrossRef]
- Guo, L.; Wang, L.; Huang, S.; Qu, J. Synthesis and properties of novel water-dispersible polyisocyanates. J. Appl. Polym. Sci. 2017, 134, 44735. [Google Scholar] [CrossRef]
- Lou, C.; Di, M. Study on cross-linking agent of a novel one-component API adhesive. J. Adhes. Sci. Technol. 2013, 27, 2340–2351. [Google Scholar] [CrossRef]
- Ma, G.; Guan, T.; Hou, C.; Wu, J.; Wang, G.; Ji, X.; Wang, B. Preparation, properties and application of waterborne hydroxyl-functional polyurethane/acrylic emulsions in two-component coatings. J. Coat. Technol. Res. 2015, 12, 505–3512. [Google Scholar] [CrossRef]
- Ji, W.; Song, W.; Zheng, Y.; He, X. Improvement of method for determination of isocyanate group content in polyurethane prepolymer. Appl. Mech. Mater. 2013, 303, 2533–2536. [Google Scholar] [CrossRef]
- Lai, X.; Shen, Y.; Wang, L.; Li, Z. Preparation and performance of waterborne polyurethane/nanosilica hybrid materials. Polym.-Plast. Technol. 2011, 50, 740–747. [Google Scholar] [CrossRef]
- Marand, A.; Dahlin, J.; Karlsson, D.; Skarping, G.; Dalene, M. Determination of technical grade isocyanates used in the production of polyurethane plastics. J. Environ. Monit. 2004, 6, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Modesti, M.; Lorenzetti, A. An experimental method for evaluating isocyanate conversion and trimer formation in polyisocyanate–polyurethane foams. Eur. Polym. J. 2001, 37, 949–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Maxted, J.; Barber, A.; Lowe, C.; Smith, R. The durability of clear polyurethane coil coatings studied by FTIR peak fitting. Polym. Degrad. Stabil. 2013, 98, 527–534. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.; Vekemans, J.; Sikkema, J. NMR based determination of minute acid functionality: End-groups in PET. Polymer 2003, 44, 4429–4434. [Google Scholar] [CrossRef]
- Moghimi, A.; Omrani, I.; Khanmiri, H.R.; Bahadorbeigi, R.; Mahmoodi, M. Determination of NCO content of the urethane prepolymers by 19F NMR spectroscopy. Polym. Test. 2014, 33, 30–33. [Google Scholar] [CrossRef]
- Ji, S.; Hoye, R.T.; Macosko, c. Primary amine quantification in Polymers: Functionality by 19F NMR Spectroscopy. Macromolecules 2005, 38, 4679–4686. [Google Scholar] [CrossRef]
- Qiu, L.; Shen, Y.; Wang, C.; Yang, X. Scanning electron microscopy analysis of guar gum in the dissolution, gelation and gel-breaking process. Polym. Test. 2018, 68, 95–99. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, L.; Liao, B.; Pang, H. Self-assembly of well-defined thermo-responsive fluoropolymer and its application in tunable wettability surface. Polymer 2014, 55, 5350–5357. [Google Scholar] [CrossRef]
- Bayer, S.; Brandi, F.; Cingolani, R.; Athanassiou, A. Modification of wetting properties of laser-textured surfaces by depositing triboelectrically charged Teflon particles. Colloid Polym. Sci. 2013, 291, 367–373. [Google Scholar] [CrossRef]











| Samples | S(-NH) | S(-N=C=O) | S(-NH):S(-N=C=O) |
|---|---|---|---|
| 1 h | 239.81 | 199.89 | 1.219 |
| 5 h | 243.16 | 196.84 | 1.235 |
| 10 h | 307.01 | 133.21 | 2.305 |
| 24 h | 369.02 | 70.87 | 5.207 |
| Samples | S(-NH) | S(-N=C=O) | S(-NH):S(-N=C=O) |
|---|---|---|---|
| 25 °C | 239.81 | 199.89 | 1.219 |
| 50 °C | 315.23 | 116.68 | 2.715 |
| 70 °C | 353.79 | 87.59 | 4.043 |
| 90 °C | 394.63 | 45.87 | 8.603 |
| Emulsion Sample | Contact Angles/° | Surface Free Energy/(mJ·m−2) | |||
|---|---|---|---|---|---|
| Water | Diiodomethane | γd | γp | γ | |
| E1 |  109.6 109.6 |  58.7 58.7 | 27.10 | 2.09 | 29.19 |
| E2 |  91.2 91.2 |  52.2 52.2 | 28.10 | 3.14 | 31.24 |
| E3 |  75.3 75.3 |  47.3 47.3 | 29.89 | 4.29 | 34.18 |
| E4 |  33.7 33.7 |  30.1 30.1 | 34.40 | 4.41 | 38.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Zhang, Y.; Long, X.; Ye, Z.; Qu, Z.; Yang, X.; Wang, C. Scanning Electron Microscopy Investigation for Monitoring the Emulsion Deteriorative Process and Its Applications in Site-Directed Reaction with Paper Fabric. Molecules 2021, 26, 6471. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216471
Qiu L, Zhang Y, Long X, Ye Z, Qu Z, Yang X, Wang C. Scanning Electron Microscopy Investigation for Monitoring the Emulsion Deteriorative Process and Its Applications in Site-Directed Reaction with Paper Fabric. Molecules. 2021; 26(21):6471. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216471
Chicago/Turabian StyleQiu, Liewei, Yongkang Zhang, Xueli Long, Zhi Ye, Zhangmingzu Qu, Xiaowu Yang, and Chen Wang. 2021. "Scanning Electron Microscopy Investigation for Monitoring the Emulsion Deteriorative Process and Its Applications in Site-Directed Reaction with Paper Fabric" Molecules 26, no. 21: 6471. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216471