Removal of Flotation Collector O-Isopropyl-N-ethylthionocarbamate from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
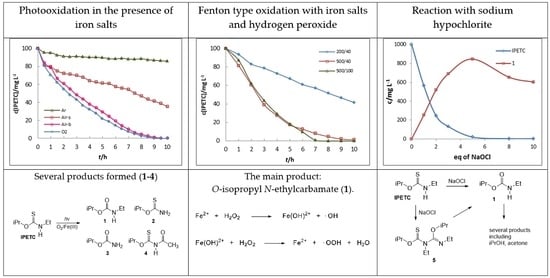
2.1. Photooxidation in the Presence of Iron Salts
2.2. Fenton Type Oxidation with Iron Salts and Hydrogen Peroxide
2.3. Reaction with Sodium Hypochlorite
3. Materials and Methods
3.1. Chemicals
3.2. Analyses of Reaction Mixtures
3.2.1. HPLC Analyses
3.2.2. GC/MS Analysis
3.2.3. Determination of Sulfate (Typical Experiment)
3.3. Degradation Experiments
3.3.1. Photochemical Degradation Experiments
3.3.2. Degradation of IPETC under Sunlight
3.3.3. Reaction with Sodium Hypochlorite
3.3.4. Reaction with FeSO4/H2O2
3.4. Syntheses and Isolation of New Compounds
3.4.1. Synthesis of O-Isopropyl N-ethylcarbamate (1)
3.4.2. Isolation of Isopropyl N-((ethylimino)(isopropoxy)methyl)-N-ethylthionocarbamate (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marabini, A.M.; Barbaro, M.; Alesse, V. New reagents in sulphide mineral flotation. Int. J. Miner. Process. 1991, 33, 291–306. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hope, G.A.; Lee, K.C.; Petrovic, E.A.; Woods, R. Adsorption of O-isopropyl-N-ethyl thionocarbamate on Cu sulfide ore minerals. Miner. Eng. 2014, 69, 120–132. [Google Scholar] [CrossRef]
- Davis, F.T.; Hyatt, D.E.; Cox, C.H. Flotation (AM Gaudin Memorial Volume); Fuerstenau, M.C., Ed.; American Institute of Mining, Metallurgical and Petroleum Engineers: New York, NY, USA, 1976; Volume 2, pp. 1307–1336. [Google Scholar]
- Guo, Z.; Yao, J.; Wang, F.; Yuan, Z.; Bararunyeretse, P.; Zhao, Y. Effect of three typical sulfide mineral flotation collectors on soil microbial activity. Environ. Sci. Pollut. Res. 2016, 23, 7425–7436. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/23653/7/3/1 (accessed on 25 January 2021).
- Vandekar, M.; Pleština, R.; Wilhelm, K. Toxicity of carbamates for mammals. Bull. Wld. Hlth. Org. 1971, 44, 241–249. [Google Scholar]
- Schlatter, J.; Lutz, W.K. The carcinogenic potential of ethyl carbamate (urethane): Risk assessment at human dietary exposure levels. Food Chem Toxicol. 1990, 28, 205–211. [Google Scholar] [CrossRef] [Green Version]
- EPA. Available online: https://www.epa.gov/haps/health-effects-notebook-hazardous-air-pollutants (accessed on 22 January 2021).
- Bu, Y.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Fundamental Flotation behaviours of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.Y.; Zhong, H.; Dai, T.G.; Xia, L.Y. Investigation of the effect of N-substituents on performance of thionocarbamates as selective collectors for copper sulfides by ab initio calculations. Miner. Eng. 2008, 21, 1050–1054. [Google Scholar] [CrossRef]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Interaction of thionocarbamate and thiourea collectors with sulphide. Int. J. Miner. Process. 1997, 50, 227–242. [Google Scholar] [CrossRef]
- Prabchakar, S.; Khangaonkar, P.R. Minerals: A flotation and adsorption study. Int. J. Miner. Process. 1997, 50, 87–95. [Google Scholar]
- Chen, S.; Gong, W.; Mei, G.; Zhou, Q.; Bai, C.; Xu, N. Primary biodegradation of sulfide mineral floatation collectors. Miner. Eng. 2011, 24, 953–955. [Google Scholar] [CrossRef]
- Chen, S.; Gong, W.; Mei, G.; Han, W. Anaerobic biodegradation of ethylthionocarbamate by the mixed bacteria under various electron acceptor conditions. Bioresource Technol. 2011, 102, 10772–10775. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gong, W.; Mei, G.; Xiong, L.; Liu, X.; Han, W.; Xu, N. Integrated assessment for aerobic biodegradability of sulfide mineral flotation collectors. Desalin. Water Treat. 2013, 51, 3125–3132. [Google Scholar] [CrossRef]
- Bavcon Kralj, M.; Černigoj, U.; Franko, M.; Trebše, P. Comparison of photocatalysis and photolysis of malathion, isomalathion, malaoxon, and commercial malathion—Products and toxicity studies. Water Res. 2007, 41, 4504–4514. [Google Scholar] [CrossRef]
- Gaelli, R.; Rich, H.W.; Scholtz, R. Toxicity of organophosphate insecticides and their metabolites to the water flea Daphnia magna, the Microtox test and an acetylcholinesterase inhibition test. Aquat. Toxicol. 1994, 30, 259–269. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Brand, N.; Mailhot, G.; Bolte, M. Degradation photoinduced by Fe(III): Method of alkylphenol ethoxylates removal in water. Environ. Sci. Technol. 1998, 32, 2715–2720. [Google Scholar] [CrossRef]
- Mazellier, P.; Mailhot, G.; Bolte, M. Photochemical behavior of the iron(III)/2,6-dimethylphenol system. New, J. Chem. 1997, 21, 389–397. [Google Scholar]
- Catastini, C.; Sarakha, M.; Mailhot, G.; Bolte, M. Iron(III) aquacomplexes as effective photocatalysts for the degradation of pesticides in homogeneous aqueous solutions. Sci. Tot. Environ. 2002, 298, 219–228. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Buterworth-Heinemann: Oxford, UK, 1998; p. 1089. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 83rd ed.; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 8–86. [Google Scholar]
- Sivakamasundari, S.; Ganesan, R. Kinetics and Mechanism of the Reaction between Phenyl Isocyanate and Alcohols in Benzene Medium. J. Org. Chem. 1984, 49, 720–722. [Google Scholar] [CrossRef]
- Walter, W.; Curts, J.; Pawelzik, H. Oxydationsprodukte sekundärer und tertiärer Thioamide. Liebigs Ann. Chem. 1961, 643, 29–38. [Google Scholar] [CrossRef]
- Needles, H.L.; Whitfield, R.E. Free-Radical Chemistry of Peptide Bonds. I. Dealkylation of Substituted Amides. J. Org. Chem. 1964, 29, 3632–3634. [Google Scholar] [CrossRef]
- Needles, H.L.; Whitfield, R.E. Free-Radical Chemistry of Peptide Bonds. II. Conversion of Lactams to Imides. J. Org. Chem. 1966, 31, 341–342. [Google Scholar]
- Doumaux, A.R.; McKeon, J.E.; Trecker, D.J. The Metal Ion Catalyzed Peroxide Oxidation of Organic Substrates. A Selective Synthesis of Imides. J. Am. Chem. Soc. 1969, 91, 3992–3993. [Google Scholar] [CrossRef]
- Hayon, E.; Ibata, T.; Lichtin, N.N.; Simic, M. Sites of Attack of Hydroxyl Radicals on Amides in Aqueous Solution. J. Am. Chem. Soc. 1970, 92, 3898–3903. [Google Scholar] [CrossRef]
- Legacy, C.J.; Wang, A.; O’Day, B.J.; Emmert, M.H. Iron-Catalyzed Cα–H Oxidation of Tertiary, Aliphatic Amines to Amides under Mild Conditions. Angew. Chem. Int. Ed. 2015, 54, 14907–14910. [Google Scholar] [CrossRef]
- Lin, W.; Tian, J.; Ren, J.; Xu, P.; Dai, Y.; Sun, S.; Wu, C. Oxidation of aniline aerofloat in flotation wastewater by sodium hypochlorite solution. Environ. Sci. Pollut. Res. Int. 2016, 23, 785–792. [Google Scholar] [CrossRef]
- Horner, L.; Gerhard, J. Der oxidative Abbau von Thiophosphin- Thiophosphon- und Thiophosphorsäureestern mit Hypochlorit. Phosphorus Sulfur. 1985, 22, 13–21. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yao, J.; Bavcon Kralj, M.; Dolenc, D.; Trebše, P. Removal of Flotation Collector O-Isopropyl-N-ethylthionocarbamate from Wastewater. Molecules 2021, 26, 6676. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216676
Wang Z, Yao J, Bavcon Kralj M, Dolenc D, Trebše P. Removal of Flotation Collector O-Isopropyl-N-ethylthionocarbamate from Wastewater. Molecules. 2021; 26(21):6676. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216676
Chicago/Turabian StyleWang, Zhe, Jun Yao, Mojca Bavcon Kralj, Darko Dolenc, and Polonca Trebše. 2021. "Removal of Flotation Collector O-Isopropyl-N-ethylthionocarbamate from Wastewater" Molecules 26, no. 21: 6676. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216676