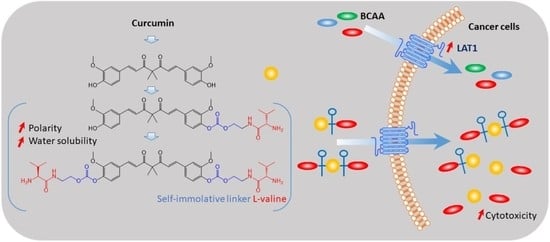
Synthesis and Characterization of the Ethylene-Carbonate-Linked L-Valine Derivatives of 4,4-Dimethylcurcumin with Potential Anticancer Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Water Solubility Analysis of DMCU, 1-TFA, and 2-TFA
2.3. Anti-Proliferative Evaluation of 1-TFA, 2-TFA, and DMCU in LAT1-Expressing Lung Cancer Cells
2.4. Evaluation of Intracellular Uptake and Bioconversion of DMCU, 1-TFA, and 2-TFA in NCI-H460 Cell Line
3. Materials and Methods
3.1. General Synthetic Methods
3.2. The Synthetic Procedures for 5, 6, 7, 8, 1-TFA and 2-TFA
3.2.1. Tert-Butyl (S)-(1-((2-Hydroxyethyl)Amino)-3-Methyl-1-Oxobutan-2-yl)Carbamate (5)
3.2.2. Tert-Butyl (S)-(3-Methyl-1-((2-(((4-Nitrophenoxy)Carbonyl)Oxy)Ethyl)Amino)-1-Oxobutan-2-yl)Carbamate (6)
3.2.3. Compound 7
3.2.4. Compound 8
3.2.5. Compound 1-TFA
3.2.6. Compound 2-TFA
3.3. Biological Assays
3.3.1. Cell Culture
3.3.2. In Vitro Antiproliferative Assay
3.4. Preparation of Standard Solution of DMCU, 1-TFA and 2-TFA
3.5. Preparation of Aqueous Solution of 1-TFA and 2-TFA for Solubility Analysis
3.6. Analysis Method of LC-MS
3.7. Cell Extracts for LC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 8, 1517–1535. [Google Scholar]
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals based chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phytother. Res. 2019, 33, 3064–3089. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernandez-Carranza, P.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic compounds: A good choice against chronic degenerative diseases. Stud. Nat. Prod. Chem. 2018, 59, 79–108. [Google Scholar]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Ashrap, P.; Zheng, G.; Wan, Y.; Li, T.; Hu, W.; Li, W.; Zhang, H.; Zhang, Z.; Hu, J. Discovery of a widespread metabolic pathway within and among phenolic xenobiotics. Proc. Natl. Acad. Sci. USA 2017, 23, 6062–6067. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.M.; Bai, C.L.; Zhang, J.; Liu, Y.; Dong, B.Y.; Zhang, Y.T.; Liu, D. In Vitro study on the interaction of 4,4-dimethylcurcumin with calf thymus DNA. J. Lumin. 2015, 166, 48–53. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Sousa, E.; Vasconcelos, M.H.; Pinto, M. Curcumin: A natural lead for potential new drug candidates. Curr. Med. Chem. 2015, 22, 4196–4232. [Google Scholar] [CrossRef]
- Liu, B.; Xia, M.; Ji, X.; Xu, L.; Dong, J. Synthesis and antiproliferative effect of novel curcumin analogues. Chem. Pharm. Bull. 2013, 61, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.S.; Wang, Q.; Sun, D.D.; Dai, F.; Zhou, B. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur. J. Med. Chem. 2017, 134, 72–85. [Google Scholar] [CrossRef]
- Yang, Y.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.T.; Chang, L.C.; Hung, H.Y.; Lin, H.Y.; Shih, M.H.; Tsai, C.H.; Kuo, S.C.; Lee, K.H. New bis(hydroxymethyl) alkanoate curcuminoid derivatives exhibit activity against triple-negative breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2017, 131, 141–151. [Google Scholar] [CrossRef]
- Chang, L.C.; Hsieh, M.T.; Yang, J.S.; Lu, C.C.; Tsai, F.J.; Tsao, J.W.; Chiu, Y.J.; Kuo, S.C.; Lee, K.H. Effect of bis(hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell cycle arrest, apoptotic and autophagic pathway in triple-negative breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int. J. Oncol. 2018, 52, 67–76. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hou, Y.C.; Yang, J.S.; Lin, H.Y.; Chang, T.Y.; Lee, K.H.; Kuo, S.C.; Hsieh, M.T. Synthesis, anticancer activity, and preliminary pharmacokinetic evaluation of 4,4-disubstituted curcuminoid 2,2-bis(Hydroxymethyl)propionate derivatives. Molecules 2020, 25, 479. [Google Scholar] [CrossRef] [Green Version]
- Barthelemy, C.; André, B. Ubiquitylation and endocytosis of the human LAT1/SLC7A5 amino acid transporter. Sci. Rep. 2019, 9, 16760. [Google Scholar] [CrossRef] [Green Version]
- Häfliger, P.; Charles, R.P. The L-Type Amino Acid Transporter LAT1-An Emerging Target in Cancer. Int. J. Mol. Sci. 2019, 20, 2428. [Google Scholar] [CrossRef] [Green Version]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Hodson, N.; Brown, T.; Joanisse, S.; Aguirre, N.; West, D.W.D.; Moore, D.R.; Baar, K.; Breen, L.; Philp, A. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients 2017, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Ogata, S.; Nakanishi, K.; Ozeki, Y.; Hiroi, S.; Tominaga, S.; Aida, S.; Matsuo, H.; Sakata, T.; Kawai, T. LAT1 expression in non-small-cell lung carcinomas: Analyses by semiquantitative reverse transcription-PCR (237 cases) and immunohistochemistry (295 cases). Lung Cancer 2010, 68, 58–65. [Google Scholar] [CrossRef]
- Kaira, K.; Oriuchi, N.; Takahashi, T.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Naito, T.; et al. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am. J. Transl. Res. 2011, 3, 468–478. [Google Scholar]
- Lopes, C.; Pereira, C.; Medeiros, R. ASCT2 and LAT1 Contribution to the hallmarks of cancer: From a molecular perspective to clinical translation. Cancers 2021, 13, 203. [Google Scholar] [CrossRef]
- Vale, N.; Ferreira, A.; Matos, J.; Fresco, P.; Gouveia, M.J. Amino acids in the development of prodrugs. Molecules 2018, 23, 2318. [Google Scholar] [CrossRef] [Green Version]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, J.; Peltokangas, S.; Gynther, M.; Natunen, T.; Hiltunen, M.; Auriola, S.; Ruponen, M.; Vellonen, K.S.; Huttunen, K.M. L-type amino acid transporter 1 (LAT1/Lat1)-utilizing prodrugs can improve the delivery of drugs into neurons, astrocytes and microglia. Sci. Rep. 2019, 9, 12860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranoy-Opalinski, I.; Fernandes, A.; Thomas, M.; Gesson, J.P.; Papot, S. Design of self-immolative linkers for tumour-activated prodrug therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 618–637. [Google Scholar] [CrossRef]
- Selwan, E.M.; Edinger, A.L. Branched chain amino acid metabolism and cancer: The importance of keeping things in context. Transl. Cancer Res. 2017, 6, S578–S584. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, H.; Kaira, K.; Oriuchi, N.; Shimizu, K.; Tominaga, H.; Yanagitani, N.; Sunaga, N.; Ishizuka, T.; Nagamori, S.; Promchan, K.; et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010, 30, 4819–4828. [Google Scholar]
- Gwinn, D.M.; Lee, A.G.; Briones-Martin-Del-Campo, M.; Conn, C.S.; Simpson, D.R.; Scott, A.I.; Le, A.; Cowan, T.M.; Ruggero, D.; Sweet-Cordero, E.A. Oncogenic KRAS regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and alters sensitivity to L-asparaginase. Cancer Cell 2018, 33, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Fernando, L.P.; Kandel, P.K.; Yu, J.; McNeill, J.; Ackroyd, P.C.; Christensen, K.A. Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles. Biomacromolecules 2010, 11, 2675–2682. [Google Scholar] [CrossRef] [Green Version]
- Semmling, M.; Kreft, O.; Muñoz Javier, A.; Sukhorukov, G.B.; Käs, J.; Parak, W.J. A novel flow-cytometry-based assay for cellular uptake studies of polyelectrolyte microcapsules. Small 2008, 4, 1763–1768. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]





| Compound | IC50 a,b (μM) | ||
|---|---|---|---|
| NCI-H460 | NCI-H358 | A549 | |
| DMCU | 2.13 ± 0.16 | 1.62 ± 0.12 | 2.50 ± 0.03 |
| 1-TFA | 1.95 ± 0.16 | 1.92 ± 0.13 | 3.17 ± 0.14 |
| 2-TFA | 1.69 ± 0.04 | 1.26 ± 0.06 | 1.99 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y.; Lin, H.-Y.; Ramasamy, M.; Kuo, S.-C.; Lee, P.-C.; Hsieh, M.-T. Synthesis and Characterization of the Ethylene-Carbonate-Linked L-Valine Derivatives of 4,4-Dimethylcurcumin with Potential Anticancer Activities. Molecules 2021, 26, 7050. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26227050
Lee D-Y, Lin H-Y, Ramasamy M, Kuo S-C, Lee P-C, Hsieh M-T. Synthesis and Characterization of the Ethylene-Carbonate-Linked L-Valine Derivatives of 4,4-Dimethylcurcumin with Potential Anticancer Activities. Molecules. 2021; 26(22):7050. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26227050
Chicago/Turabian StyleLee, Der-Yen, Hui-Yi Lin, Manickavasakam Ramasamy, Sheng-Chu Kuo, Pei-Chih Lee, and Min-Tsang Hsieh. 2021. "Synthesis and Characterization of the Ethylene-Carbonate-Linked L-Valine Derivatives of 4,4-Dimethylcurcumin with Potential Anticancer Activities" Molecules 26, no. 22: 7050. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26227050