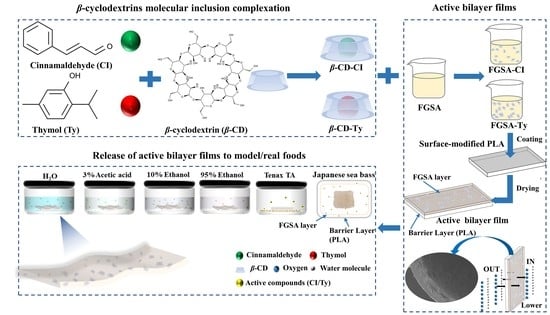
Release of Cinnamaldehyde and Thymol from PLA/Tilapia Fish Gelatin-Sodium Alginate Bilayer Films to Liquid and Solid Food Simulants, and Japanese Sea Bass: A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantification of the Active Compounds in β-CD Inclusion Complexes
2.2. FTIR Analysis
2.3. Morphology of Films
2.4. Thickness
2.5. Water Contact Angle (WCA)
2.6. Release of Active Compounds into Food Simulants and Real Foods
2.6.1. Release of Active Compounds into Liquid Food Simulants
2.6.2. Release of Active Compounds into Tenax TA
2.6.3. Release of Active Compounds into Sea Bass Fillet
2.7. Antibacterial and Antioxidant Activities of Films
2.7.1. Antibacterial Activity
2.7.2. Antioxidant Activity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Active Bilayer PLA-FGSA Films
3.2.1. Encapsulation of Active Compounds in β-Cyclodextrin
3.2.2. Surface Modification of PLA Layer
3.2.3. Preparation of Tilapia Fish Gelatin-Sodium Alginate Coating Solution
3.2.4. Coating Process
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM)
3.4. Thickness
3.5. Water Contact Angle (WCA)
3.6. Release of Active Compounds into Food Simulants and Real Food
3.6.1. Release Test of Active Compounds into Liquid Food Simulants
3.6.2. Mathematical Models of Liquid Food Simulants
3.6.3. Release Test of Active Compounds into Solid Dry Food Simulant
3.6.4. Release Test of Active Compounds into Sea Bass Fillets
3.6.5. HPLC Analysis
3.7. Antibacterial and Antioxidant Activities of Films
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced mechanical and antioxidant properties of biodegradable poly (lactic) acid-poly(3-hydroxybutyrate-co-4-hydroxybutyrate) film utilizing α-tocopherol for peach storage. Packag. Technol. Sci. 2021, 34, 187–199. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Song, W.; Du, Y.; Yang, C.; Li, L.; Wang, S.; Liu, Y.; Wang, W. Development of PVA/EVA-based bilayer active film and its application to mutton. LWT-Food Sci. Technol. 2020, 133, 110109. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; McDonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in beta-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by betacyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Z.; Li, R. Complexation and molecular microcapsules of Litsea cubeba essential oil with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 2009, 228, 865–873. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Han, W.; Ma, Y.; Shao, Z.; Li, L. Development of Double-Layer Active Films Containing Pomegranate Peel Extract for the Application of Pork Packaging. J. Food Process. Eng. 2017, 40, e12388. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, L.; Dong, Q.; Kang, Y.; Osako, K.; Li, L. Characterization of PLA-P3,4HB active film incorporated with essential oil: Application in peach preservation. Food Chem. 2020, 313, 126134. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Miao, L.; Walton, W.C.; Wang, L.; Li, L.; Wang, Y. Characterization of polylactic acids-polyhydroxybutyrate based packaging film with fennel oil, and its application on oysters. Food Packag. Shelf Life 2019, 22, 100388. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Heising, J.; Dekker, M. Multiresponse kinetic modelling of the formation, release, and degradation of allyl isothiocyanate from ground mustard seeds to improve active packaging. J. Food Eng. 2021, 292, 110370. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Mahfoudh, R.; Moundanga, S.; Brachais, C.-H.; Chambin, O.; Debeaufort, F. Modeling of the release kinetics of phenolic acids embedded in gelatin/chitosan bioactive-packaging films: Influence of both water activity and viscosity of the food simulant on the film structure and antioxidant activity. Int. J. Biol. Macromol. 2020, 160, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) No. 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. European Union, Brussels. Off. J. Eur. Commun. 2011, L12, 1–89. [Google Scholar]
- Huang, Z.; Jia, S.; Zhang, L.; Liu, X.; Luo, Y. Inhibitory effects and membrane damage caused to fish spoilage bacteria by cinnamon bark (Cinnamomum tamala) oil. LWT-Food Sci. Technol. 2019, 112, 108195. [Google Scholar] [CrossRef]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of antimicrobial active film containing CINnamaldehyde and its application to snakehead (Ophiocephalus argus) fish. J. Food Process. Eng. 2017, 40, e12554. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Herrera, A.; Rodríguez, F.J.; Bruna, J.E.; Abarca, R.L.; Galotto, M.J.; Guarda, A.; Mascayano, C.; Sandoval-Yáñez, C.; Padula, M.; Felipe, F.R.S. Antifungal and physicochemical properties of inclusion complexes based on β-cyclodextrin and essential oil derivatives. Food Res. Int. 2019, 121, 127–135. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT–Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Chen, Y.; Mensah, A.; Wang, Q.; Li, D.; Qiu, Y.; Wei, Q. Hierarchical porous nanofibers containing thymol/beta-cyclodextrin: Physico-chemical characterization and potential biomedical applications. Mater. Sci. Eng. C 2020, 115, 111155. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.G.G.; Thrivikraman, G.; Menezes, P.P.; Franca, C.M.; Lima, B.S.; Carvalho, Y.; Souza, E.; Duarte, M.C.; Shanmugam, S.; Quintans-Junior, L.J.; et al. Carvacrol/beta-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy protein–Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Siriprom, W.; Sangwaranatee, N.; Herman; Chantarasunthon, K.; Teanchai, K.; Chamchoi, N. Characterization and analyzation of the poly (L-lactic acid) (PLA) films. Mater. Today: Proc. 2018, 5, 14803–14806. [Google Scholar] [CrossRef]
- Ho, T.C.; Kim, M.H.; Cho, Y.-J.; Park, J.-S.; Nam, S.Y.; Chun, B.-S. Gelatin-sodium alginate based films with Pseuderanthemum palatiferum (Nees) Radlk. freeze-dried powder obtained by subcritical water extraction. Food Packag. Shelf Life 2020, 24, 100469. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Javidi, Z.; Rezaei, M. Efficient gas barrier properties of multi-layer films based on poly(lactic acid) and fish gelatin. Int. J. Biol. Macromol. 2016, 92, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Liu, Z.; Guo, X.; Wu, Q.; Qiao, L. Controlled Release System by Active Gelatin Film Incorporated with β-Cyclodextrin-Thymol Inclusion Complexes. Food Bioprocess Technol. 2018, 11, 1695–1702. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/thermal properties and heat seal ability of bilayer films based on fish gelatin and poly(lactic acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ji, M.; Wang, J.; Cheng, C.; Chen, L.; Wen, C.; Zhang, Q. Physicochemical, Antioxidant, In Vitro Release, and Heat Sealing Properties of Fish Gelatin Films Incorporated with β-Cyclodextrin/Curcumin Complexes for Apple Juice Preservation. Food Bioprocess Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Zhou, W. The preparation, characterization, anti-ultraviolet and antimicrobial activity of gelatin film incorporated with berberine-HP-β-CD. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124273. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Turila, A.; Apjok, R.; Ciocian, A.; Mihaly Cozmuta, L.; Peter, A.; Nicula, C.; Galić, N.; Benković, T. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015, 49, 144–155. [Google Scholar] [CrossRef]
- Ye, Y.; Zhu, M.; Miao, K.; Li, X.; Li, D.; Mu, C. Development of Antimicrobial Gelatin-Based Edible Films by Incorporation of Trans-Anethole/β-Cyclodextrin Inclusion Complex. Food Bioprocess Technol. 2017, 10, 1844–1853. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Release kinetics of carvacrol and eugenol from poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) films for food packaging applications. Eur. Polym. J. 2017, 92, 185–193. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R.; Jindal, D. RSM-CCD optimized microwave-assisted synthesis of chitosan and gelatin-based pH sensitive, inclusion complexes incorporated hydrogels and their use as controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 48, 161–173. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Soto-Valdez, H.; González-León, A.; Álvarez-Parrilla, E.; Martín-Belloso, O.; González-Aguilar, G.A. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2008, 60, 359–368. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Structural relaxation and glass transition in high-solid gelatin systems crosslinked with genipin. Int. J. Biol. Macromol. 2019, 141, 867–875. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Requena, R.; Vargas, M.; Chiralt, A. Study of the potential synergistic antibacterial activity of essential oil components using the thiazolyl blue tetrazolium bromide (MTT) assay. LWT-Food Sci. Technol. 2019, 101, 183–190. [Google Scholar] [CrossRef]
- Bernardos, A.; Bozik, M.; Alvarez, S.; Saskova, M.; Perez-Esteve, E.; Kloucek, P.; Lhotka, M.; Frankova, A.; Martinez-Manez, R. The efficacy of essential oil components loaded into montmorillonite against Aspergillus niger and Staphylococcus aureus. Flavour Fragr. J. 2019, 34, 151–162. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, C.H.; Yin, S.W.; Yang, X.Q. Development and characterization of novel antimicrobial bilayer films based on Polylactic acid (PLA)/Pickering emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, S.; Zhou, W.; Chen, Y.; Zhang, B.; Guo, Y. Effect of morin-HP-β-CD inclusion complex on anti-ultraviolet and antioxidant properties of gelatin film. React. Funct. Polym. 2019, 137, 140–146. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Son, S.M.; Hong, S.I. Characterization of protein-coated polypropylene films as a novel composite structure for active food packaging application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, J.; Jiang, S.; Lin, L. Effect of sodium alginate on physicochemical properties of fish skin gelatin films. Shipin Kexue/Food Sci. 2019, 40, 81–86. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Crank, J. Food Additives Permitted for Direct Addition to Food for Human Consumption: Synthetic Flavoring Substances and Adjuvants; Clarendon Press: London, UK, 1975. [Google Scholar]






| β-CD Inclusion Complexes | Process Efficiency (w/w %) | Entrapment Efficiency (w/w %) | Drug Loading (w/w %) |
|---|---|---|---|
| β-CD-CI | 79.98 ± 0.04 b | 88.82 ± 0.04 b | 11.57 ± 0.00 b |
| β-CD-Ty | 62.35 ± 0.01 a | 50.46 ± 0.01 a | 9.65 ± 0.09 a |
| Film Type | WCA (°) | Image |
|---|---|---|
| PLA | 71.90 ± 1.55 a |  |
| PLA/FGSA | 85.55 ± 2.88 b |  |
| PLA/FGSA-CI | 106.74 ± 6.35 c |  |
| PLA/FGSA-Ty | 108.21 ± 6.43 c |  |
| Active Compounds | Simulants | α | k | R2 | D (m2 s−1) |
|---|---|---|---|---|---|
| CI | water | 0.11 | 0.26 | 0.96 | 2.16 × 10−13 |
| 3% acetic acid | 0.12 | 0.22 | 0.92 | 1.77 × 10−13 | |
| 10% ethanol | 0.087 | 0.34 | 0.93 | 2.96 × 10−13 | |
| 95% ethanol | 0.13 | 0.18 | 0.96 | 1.88 × 10−13 | |
| Ty | water | 0.090 | 0.32 | 0.97 | 3.31 × 10−13 |
| 3% acetic acid | 0.13 | 0.19 | 0.91 | 1.05 × 10−13 | |
| 10% ethanol | 0.091 | 0.32 | 0.94 | 3.61 × 10−13 | |
| 95% ethanol | 0.15 | 0.15 | 0.96 | 2.67 × 10−13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, Y.; Shi, W.; Zheng, H.; Wang, L.; Li, L. Release of Cinnamaldehyde and Thymol from PLA/Tilapia Fish Gelatin-Sodium Alginate Bilayer Films to Liquid and Solid Food Simulants, and Japanese Sea Bass: A Comparative Study. Molecules 2021, 26, 7140. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26237140
Chen J, Li Y, Shi W, Zheng H, Wang L, Li L. Release of Cinnamaldehyde and Thymol from PLA/Tilapia Fish Gelatin-Sodium Alginate Bilayer Films to Liquid and Solid Food Simulants, and Japanese Sea Bass: A Comparative Study. Molecules. 2021; 26(23):7140. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26237140
Chicago/Turabian StyleChen, Jingwen, Yinxuan Li, Wenzheng Shi, Hui Zheng, Li Wang, and Li Li. 2021. "Release of Cinnamaldehyde and Thymol from PLA/Tilapia Fish Gelatin-Sodium Alginate Bilayer Films to Liquid and Solid Food Simulants, and Japanese Sea Bass: A Comparative Study" Molecules 26, no. 23: 7140. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26237140