A Luminescent Guest@MOF Nanoconfined Composite System for Solid-State Lighting
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Structure of RhB@ZIF-8
2.2. Photophysical Properties of RhB@ZIF-8
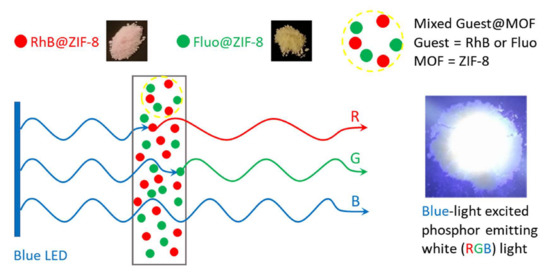
2.3. A Hybrid Guest@MOF Solid-State White Light Emitting Device (WLED)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials Synthesis
3.3. Materials Characterization
3.4. Fabrication of the WLED Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Y.; Wang, X.; Zhang, Y.; Li, Y.; Yao, X. Optical temperature sensing of up-conversion luminescent materials: Fundamentals and progress. J. Alloys Compd. 2020, 817, 152691. [Google Scholar] [CrossRef]
- Yoon, H.; Park, M.; Kim, J.; Novak, T.G.; Lee, S.; Jeon, S. Toward highly efficient luminescence in graphene quantum dots for optoelectronic applications. Chem. Phys. Rev. 2021, 2, 031303. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, B.Z. Near-infrared luminescent probes for bioimaging and biosensing. Chem. Sci. 2021, 12, 3377–3378. [Google Scholar] [CrossRef]
- Yuce, H.; Guner, T.; Dartar, S.; Kaya, B.U.; Emrullahoglu, M.; Demir, M.M. BODIPY-based organic color conversion layers for WLEDs. Dyes Pigments 2020, 173, 107932. [Google Scholar] [CrossRef]
- Yuce, H.; Guner, T.; Balci, S.; Demir, M.M. Phosphor-based white LED by various glassy particles: Control over luminous efficiency. Opt. Lett. 2019, 44, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Senoh, M.; Iwasa, N.; Nagahama, S.-i.; Yamada, T.; Mukai, T. Superbright Green InGaN Single-Quantum-Well-Structure Light-Emitting Diodes. Jpn. J. Appl. Phys. 1995, 34, L1332–L1335. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. A new phosphor for flying-spot cathode-ray tubes for color television: Yellow-emitting Y3Al5O12−Ce3+. Appl. Phys. Lett. 1967, 11, 53–55. [Google Scholar] [CrossRef]
- Bando, K.; Sakano, K.; Noguchi, Y.; Shimizu, Y. Development of High-bright and Pure-white LED Lamps. J. Light Vis. Environ. 1998, 22, 2–5. [Google Scholar] [CrossRef]
- Sakuma, K.; Omichi, K.; Kimura, N.; Ohashi, M.; Tanaka, D.; Hirosaki, N.; Yamamoto, Y.; Xie, R.-J.; Suehiro, T. Warm-white light-emitting diode with yellowish orange SiAlON ceramic phosphor. Opt. Lett. 2004, 29, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Li, Y.; Tsung, C.-K.; Li, J. Encapsulation of yellow phosphors into nanocrystalline metal–organic frameworks for blue-excitable white light emission. Chem. Commun. 2019, 55, 10669–10672. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Xu, X.; Li, J.; Song, J.; Lan, S.; Liu, S.; Cai, B.; Han, B.; Precht, J.T.; et al. Efficient and bright white light-emitting diodes based on single-layer heterophase halide perovskites. Nat. Photonics 2021, 15, 238–244. [Google Scholar] [CrossRef]
- Ogi, T.; Kaihatsu, Y.; Iskandar, F.; Wang, W.-N.; Okuyama, K. Facile Synthesis of New Full-Color-Emitting BCNO Phosphors with High Quantum Efficiency. Adv. Mater. 2008, 20, 3235–3238. [Google Scholar] [CrossRef]
- Lin, C.; Yu, M.; Cheng, Z.; Zhang, C.; Meng, Q.; Lin, J. Bluish-White Emission from Radical Carbonyl Impurities in Amorphous Al2O3 Prepared via the Pechini-Type Sol−Gel Process. Inorg. Chem. 2008, 47, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, N.; Lin, C.C.; Hu, S.-F.; Liu, R.-S. A rare earth-free GaZnON phosphor prepared by combustion for white light-emitting diodes. J. Mater. Chem. B 2015, 3, 1473–1479. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Liu, H.; Lin, J.; Xu, X.; Meng, F.; Zhao, J.; Tang, C. Blue emitting BCNO phosphors with high quantum yields. J. Mater. Chem. B 2015, 3, 3311–3317. [Google Scholar] [CrossRef]
- Nyalosaso, J.L.; Boonsin, R.; Vialat, P.; Boyer, D.; Chadeyron, G.; Mahiou, R.; Leroux, F. Towards rare-earth-free white light-emitting diode devices based on the combination of dicyanomethylene and pyranine as organic dyes supported on zinc single-layered hydroxide. Beilstein J. Nanotechnol. 2019, 10, 760–770. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, B.; Hu, X.; Wei, Y.; Zhang, C.; An, B.; Wang, C.; Lin, W. Warm-White-Light-Emitting Diode Based on a Dye-Loaded Metal-Organic Framework for Fast White-Light Communication. ACS Appl. Mater. Interfaces 2017, 9, 35253–35259. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Tan, J.C. Dual-Guest Functionalized Zeolitic Imidazolate Framework-8 for 3D Printing White Light-Emitting Composites. Adv. Opt. Mater. 2020, 8, 1901912. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, C.; Van der Auweraer, M.; Hofkens, J.; Tan, J.-C. Electroluminescent Guest@MOF Nanoparticles for Thin Film Optoelectronics and Solid-State Lighting. Adv. Opt. Mater. 2020, 8, 2000670. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.-Y.; Mo, J.-T.; Fu, P.-Y.; Zhao, Y.-W.; Yin, S.-Y.; Jiang, J.-J.; Pan, M.; Su, C.-Y. White-Light Emission from Dual-Way Photon Energy Conversion in a Dye-Encapsulated Metal–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 9752–9757. [Google Scholar] [CrossRef]
- Etchart, I.; Hernández, I.; Huignard, A.; Bérard, M.; Gillin, W.P.; Curry, R.J.; Cheetham, A.K. Efficient oxide phosphors for light upconversion; green emission from Yb3+ and Ho3+ co-doped Ln2BaZnO5 (Ln = Y., Gd). J. Mater. Chem. 2011, 21, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Donà, L.; Gutiérrez, M.; Möslein, A.F.; Babal, A.S.; Amin, N.; Civalleri, B.; Tan, J.-C. Tunable Fluorescein-Encapsulated Zeolitic Imidazolate Framework-8 Nanoparticles for Solid-State Lighting. ACS Appl. Nano Mater. 2021, 4, 10321–10333. [Google Scholar] [CrossRef]
- Babal, A.S.; Souza, B.E.; Möslein, A.F.; Gutiérrez, M.; Frogley, M.D.; Tan, J.-C. Broadband Dielectric Behavior of an MIL-100 Metal–Organic Framework as a Function of Structural Amorphization. ACS Appl. Electron. Mater. 2021, 3, 1191–1198. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Zhang, Y.; Gutiérrez, M.; Chaudhari, A.K.; Tan, J.-C. Dye-Encapsulated Zeolitic Imidazolate Framework (ZIF-71) for Fluorochromic Sensing of Pressure, Temperature, and Volatile Solvents. ACS Appl. Mater. Interfaces 2020, 12, 37477–37488. [Google Scholar] [CrossRef]
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Karmakar, A.; Samanta, P.; Dutta, S.; Ghosh, S.K. Fluorescent “Turn-on” Sensing Based on Metal-Organic Frameworks (MOFs). Chem.-Asian J. 2019, 14, 4506–4519. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titov, K.; Eremin, D.B.; Kashin, A.S.; Boada, R.; Souza, B.E.; Kelley, C.S.; Frogley, M.D.; Cinque, G.; Gianolio, D.; Cibin, G.; et al. OX-1 Metal–Organic Framework Nanosheets as Robust Hosts for Highly Active Catalytic Palladium Species. ACS Sustain. Chem. Eng. 2019, 7, 5875–5885. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.-H.; Chou, L.-Y.; Liu, D.-Y.; Weerapana, E.; Tsung, C.-K. Optimized Metal–Organic-Framework Nanospheres for Drug Delivery: Evaluation of Small-Molecule Encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.E.; Donà, L.; Titov, K.; Bruzzese, P.; Zeng, Z.; Zhang, Y.; Babal, A.S.; Möslein, A.F.; Frogley, M.D.; Wolna, M.; et al. Elucidating the Drug Release from Metal–Organic Framework Nanocomposites via In Situ Synchrotron Microspectroscopy and Theoretical Modeling. ACS Appl. Mater. Interfaces 2020, 12, 5147–5156. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Dye encapsulated hierarchical porous zeolitic imidazolate frameworks for carbon dioxide adsorption. J. Environ. Chem. Eng. 2020, 8, 104008. [Google Scholar] [CrossRef]
- Wang, K.; Qian, M.; Qi, H.; Gao, Q.; Zhang, C. Multifunctional zeolitic imidazolate framework-8 for real-time monitoring ATP fluctuation in mitochondria during photodynamic therapy. Nanoscale 2020, 12, 15663–15669. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wu, X.-H.; Mao, S.; Tao, W.-Q.; Li, Z. Highly luminescent sensing for nitrofurans and tetracyclines in water based on zeolitic imidazolate framework-8 incorporated with dyes. Talanta 2019, 204, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Xing, K.; Li, Y.; Tsung, C.-K.; Li, J. Three Models to Encapsulate Multicomponent Dyes into Nanocrystal Pores: A New Strategy for Generating High-Quality White Light. J. Am. Chem. Soc. 2019, 141, 14807–14813. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Kim, H.J.; Han, I.; Tan, J.C. Optochemically Responsive 2D Nanosheets of a 3D Metal-Organic Framework Material. Adv. Mater. 2017, 29, 1701463. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.K.; Han, I.; Tan, J.C. Multifunctional Supramolecular Hybrid Materials Constructed from Hierarchical Self-Ordering of In Situ Generated Metal-Organic Framework (MOF) Nanoparticles. Adv. Mater. 2015, 27, 4438–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing fluorescence lifetime standards using two-photon excitation and time-domain instrumentation: Rhodamine B, coumarin 6 and lucifer yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, T.; Zhang, Y.; Amin, N.; Tan, J.-C. A Luminescent Guest@MOF Nanoconfined Composite System for Solid-State Lighting. Molecules 2021, 26, 7583. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247583
Xiong T, Zhang Y, Amin N, Tan J-C. A Luminescent Guest@MOF Nanoconfined Composite System for Solid-State Lighting. Molecules. 2021; 26(24):7583. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247583
Chicago/Turabian StyleXiong, Tao, Yang Zhang, Nader Amin, and Jin-Chong Tan. 2021. "A Luminescent Guest@MOF Nanoconfined Composite System for Solid-State Lighting" Molecules 26, no. 24: 7583. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247583