Prenylated Trans-Cinnamic Esters and Ethers against Clinical Fusarium spp.: Repositioning of Natural Compounds in Antimicrobial Discovery
Abstract
:1. Introduction
2. Results
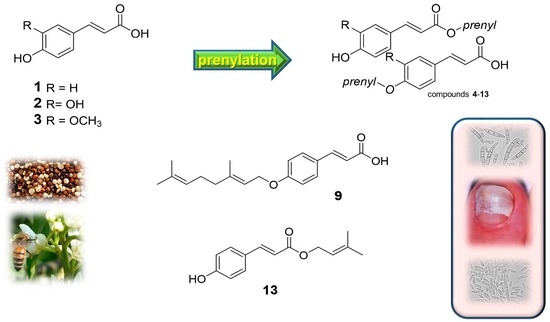
2.1. Chemistry
2.2. Antifungal Activity of the Parent Compounds 1–3 and Derivatives
2.3. Determination of the Minimal Inhibitory Concentration (MIC) and Lethal Dose 50 (LD50) for p-Coumaric acid 3,3′-Dimethyl Allyl Ester 13
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis
4.1.1. General
4.1.2. General Procedure for the Synthesis of Compounds 10–13
4.1.3. General Procedure for the Synthesis of Compounds 14–17
4.1.4. Procedure for the Synthesis of Compound 13 with Lipase
4.1.5. General Procedure for the Synthesis of Compounds 4, 6, 7, and 9
4.1.6. Synthesis of Compound 5
4.2. Fungal Strains and Culture
4.3. Evaluation of the Antifungal Activity of Compounds 1–13 in FMM Solid Medium
4.4. Evaluation of the Antifungal Activity of p-Coumaric Acid 3,3′-Dimethyl Allyl Ester (13) in FMM Liquid Medium
4.5. Optical Microscopy Examination
4.6. Data Acquisition and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Statement
Sample Availability
References
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic phytochemicals in cancer prevention and therapy: Bioavailability versus bioefficacy. J. Agric. Food Chem. 2017, 65, 7228–7239. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M.; Quave, C.L. Opportunities for plant natural products in infection control. Curr. Opin. Microbiol. 2018, 45, 89–194. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; Dantas dos Santos, W.; Polimeni Constantin, R.; Barbosa de Lima, R.; Soares, A.R.; Finger-Teixeira, A.; Rodrigues Mota, T.; Matias de Oliveira, D.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Serafim, T.L.; Milhazes, N.; Borges, F.; Oliveira, P.J. Chapter 74—Caffeic and ferulic acid derivatives: Use in Breast Cancer. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 663–671. [Google Scholar]
- Furlong, E.B.; Furlong, V.B.; Kupski, L.; Scaglioni, P.T.; de Souza, T.D.; Christ-Ribeiro, A. Use of natural resources from Southern Brazil as a strategy to mitigate fungal contamination. Crit. Rev. Food Sci. Nutr. 2020, 61, 275–282. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazz, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Muccilli, V.; Cardullo, N.; Spatafora, C.; Tringali, C. Chemoenzymatic synthesis and α-Glucosidase inhibitory activity of dimeric neolignans inspired by magnolol. J. Nat. Prod. 2017, 80, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.S. The Multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and curcumin-like molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [Green Version]
- De, P.; Yoya, G.K.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffé, M.; Baltas, M. Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef]
- Thomas, G.P.L.; Chapelon, J.Y.; Birer, A.; Inserra, C.; Lafon, C. Confocal lens focused piezoelectric lithotripter. Ultrasonics. 2020, 103, 106066. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; Feng, P.; Ahmed, S.; Sudhadham, M.; Bunyaratavej, S.; de Hoog, G.S. Spectrum of Fusarium infections in tropical dermatology evidenced by multilocus sequencing typing diagnostics. Mycoses 2015, 58, 48–57. [Google Scholar] [CrossRef]
- Guarro, J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1491–1500. [Google Scholar] [CrossRef]
- Migheli, Q.; Balmas, V.; Harak, H.; Sanna, S.; Scherm, B.; Aoki, T.; O’Donnell, K. Molecular phylogenetic diversity of dermatologic and other human pathogenic Fusaria from hospitals in Northern and Central Italy. J. Clin. Microbiol. 2010, 48, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C.; et al. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A. Fusarium onychomycosis: Prevalence, clinical presentations, response to itraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int. J. Dermatol. 2015, 54, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Cruz-Aguilar, P.; Ponce, R.M. Onychomycosis by molds. Report of 78 cases. Eur. J. Dermatol. 2007, 17, 70–72. [Google Scholar] [PubMed]
- Nucci, M.; Anaissie, E.J. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current trends in diagnosis and treatment. Am. Fam. Physician 2013, 88, 762–770. [Google Scholar]
- Malay, D.S.; Yi, S.; Borowsky, P.; Downey, M.S.; Mlodzienski, A.J. Efficacy of debridement alone versus debridement combined with topical antifungal nail lacquer for the treatment of pedal onychomycosis: A randomized, controlled trial. J. Foot Ankle Surg. 2009, 48, 294–308. [Google Scholar] [CrossRef]
- Antifungal drugs. Treat Guidel Med Lett. 2009, 7, 95–102. Available online: https://secure.medicalletter.org/TG-article-88a (accessed on 24 December 2020).
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives—Mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.; van Diepeningen, A.D.; Curfs-Breuker, I.; Hoog, G.S.de.; Meis, J.F.G.M. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J. Antimicrob. Chemother. 2015, 70, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, M.; Kong, F.; Chen, S.; Dou, H.T.; Sorrell, T.; Li, R.Y.; Xu, Y.C. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridization, and an in vitro antifungal susceptibility study. J. Clin. Microbiol. 2011, 49, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Azor, M.; Gene, J.; Cano, J.; Manikandan, P.; Venkatapathy, N.; Guarro, J. Less-frequent Fusarium species of clinical interest: Correlation between morphological and molecular identification and antifungal susceptibility. J. Clin. Microbiol. 2009, 47, 1463–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Denning, D.W.; Walsh, T.J. Future research priorities in fungal resistance. J. Infect. Dis. 2017, 216, S484–S492. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Abdullahi, M.I.; Uba, A.; Umar, A. Prenylation of aromatic secondary metabolites: A new frontier for development of novel drugs. Trop. J. Pharm. Res. 2014, 13, 307–314. [Google Scholar] [CrossRef]
- Espinoza, L.; Taborga, L.; Díaz, K.; Olea, A.F.; Cortés, H.P. Synthesis of linear geranylphenols and their effect on mycelial growth of plant pathogen Botrytis cinerea. Molecules 2014, 19, 1512–1526. [Google Scholar] [CrossRef] [Green Version]
- Genovese, S.; Ashida, H.; Yamashita, Y.; Nakgano, T.; Ikeda, M.; Daishi, S.; Epifano, F.; Taddeo, V.A.; Fiorito, S. The interaction of auraptene and other oxyprenylated phenylpropanoids with glucose transporter type 4. Phytomedicine 2017, 32, 74–79. [Google Scholar] [CrossRef]
- Genovese, S.; Fiorito, S.; Epifano, F.; Taddeo, V.A. A novel class of emerging anticancer compounds: Oxyprenylated secondary metabolites from plants and fungi. Curr. Med. Chem. 2015, 22, 3426–3433. [Google Scholar] [CrossRef]
- Shi, S.; Fan, D.; Xiang, H.; Li, H. Effective synthesis of magnetic porous molecularly imprinted polymers for efficient and selective extraction of cinnamic acid from apple juices. Food Chem. 2017, 237, 198–204. [Google Scholar] [CrossRef]
- Timokhin, V.I.; Regner, M.; Motagamwala, A.H.; Sener, C.; Karlen, S.D.; Dumesic, J.A.; Ralph, J. Production of p-Coumaric Acid from Corn GVL-Lignin. ACS Sustain. Chem. Eng. 2020, 8, 17427–17438. [Google Scholar] [CrossRef]
- Vargas-Tah, A.; Gosset, G. Production of cinnamic and p-hydroxycinnamic acids in engineered microbes. Front. Bioeng. Biotechnol. 2015, 3, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrone, V.; Genovese, S.; Carlucci, M.; Tiecco, M.; Germani, R.; Preziuso, F.; Epifano, F.; Carlucci, G.; Taddeo, V.A. A green deep eutectic solvent dispersive liquid-liquid micro-extraction (DES-DLLME) for the UHPLC-PDA determination of oxyprenylated phenylpropanoids in olive, soy, peanuts, corn, and sunflower oil. Food Chem. 2018, 245, 578–585. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on propolis from mediterranean countries: Chemical composition, biological activities and application fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef] [PubMed]
- Bodet, C.; Epifano, F.; Genovese, S.; Curini, M.; Grenier, D. Effects of 3-(40-geranyloxy-30-methoxyphenyl)-2-trans propenoic acid and its ester derivatives on biofilm formation by two oral pathogens, Porphyromonas gingivalis and Streptococcus mutans. Eur. J. Med. Chem. 2008, 43, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement Alternat. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Veiga, F.F.; Costa, M.I.; Cótica, E.S.K.; Estivalet Svidzinski, T.I.; Negri, M. Propolis for the treatment of onychomycosis. Indian J. Dermatol. 2018, 63, 515–517. [Google Scholar]
- Galletti, J.; Tobaldini-Valerio, F.K.; Silva, S.; Kioshima, É.S.; Trierveiler-Pereira, L.; Bruschi, M.; Negri, M.; Estivalet Svidzinski, T.I. Antibiofilm activity of propolis extract on Fusarium species from onychomycosis. Future Microbiol. 2017, 12, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Oufensou, S.; Scherm, B.; Pani, G.; Balmas, V.; Fabbri, D.; Dettori, M.A.; Carta, P.; Malbrán, I.; Migheli, Q.; Delogu, G. Honokiol, magnolol and its monoacetyl derivative show strong anti-fungal effect on Fusarium isolates of clinical relevance. PLoS ONE 2019, 14, e0221249. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Buitrago, M.J.; Monzon, A.; Rodriguez-Tudela, J.L. Head-to-head comparison of the activities of currently available antifungal agents against 3378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 2006, 50, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Kischkel, B.; Souza, G.K.; Chiavelli, L.U.R.; Pomini, A.M.; Estivalet Svidzinski, T.I.; Negri, M. The ability of farnesol to prevent adhesion and disrupt Fusarium keratoplasticum biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Drogari-Apiranthitou, M.; Foteini-Despina, M.; Skiada, A.; Kanioura, L.; Grammatikou, M.; Vrioni, G.; Mitroussia-Ziouva, A.; Tsakris, A.; Petrikkos, G. In vitro antifungal susceptibility of filamentous fungi causing rare infections: Synergy testing of amphotericin B, posaconazole and anidulafungin in pairs. J. Antimicrob. Chemother. 2012, 67, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Taj-Aldeen, S.J. Reduced multidrug susceptibility profile is a common feature of opportunistic Fusarium species: Fusarium multi-drug resistant pattern. J. Fungi 2017, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Chen, D.; Li, B.; Zhang, B.; Fang, M.; Zhou, L. Bioactivity and structure-activity relationship of cinnamic acid esters and their derivatives as potential antifungal agents for plant protection. PLoS ONE 2017, 12, e0176189. [Google Scholar] [CrossRef]
- Bernal, F.A.; Kaiser, M.; Wünsch, B.; Schmidt, T.J. Structure-activity relationships of cinnamate ester analogues as potent antiprotozoal agents. Chem. Med. Chem. 2020, 15, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulsk, M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [Green Version]
- Cuicui, J.T.; Huasheng, Y.; Dayong, F.; Zhoujun, S.; Wang, H.J.; Yongjiang, W.; Quing, G. Ethyl acetate as a co-solvent and sacrificial ester in the aluminum triiodide promoted chemoselective demethylation of methyl vanillate. Tetrahedron Lett. 2017, 58, 3522–3524. [Google Scholar]
- Costantin, M.A.; Conrad, J.; Beifuss, U. Laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives. Green Chem. 2012, 14, 2375–2379. [Google Scholar] [CrossRef]
- Kumar, S.S.; Begum, A.S.; Hira, K.; Niazi, S.; Kumar, B.R.P.; Araya, H.; Fujimoto, Y. Structure-based design and synthesis of new 4-methylcoumarin-based lignans as pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) inhibitors. Bioorg. Chem. 2019, 89, 102991. [Google Scholar] [CrossRef]
- Eicher, T.; Ott, M.; Speicher, A. Bryophyte Constituents; 7: New synthesis of (+)-rosmarinic acid and related compounds. Synthesis 1996, 6, 755–762. [Google Scholar] [CrossRef]
- Jaiswal, R.; Dickman, M.H.; Kuhnert, N. First diastereoselective synthesis of methyl caffeoyl-and feruloyl-muco-quinates. Org. Biomol. Chem. 2012, 10, 5266–5527. [Google Scholar] [CrossRef] [PubMed]
- Curini, M.; Epifano, F.; Genovese, S. Synthesis of a novel prodrug of 3-(40-geranyloxy-30-methoxyphenyl)-2-trans-propenoic acid for colon delivery. Bioorg. Med. Chem. Lett. 2005, 15, 5049–5052. [Google Scholar] [CrossRef]
- Griffith, D.R.; Botta, L.; St. Denis, T.G.; Snyder, S.A. Explorations of caffeic acid derivatives: Total syntheses of rufescenolide, yunnaneic acids C and D, and studies toward yunnaneic acids A and B. J. Org. Chem. 2014, 79, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Xue, C.; Peng, Y.; Katan, T.; Kistler, H.C.; Xu, J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant Microbe Interact. 2012, 15, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Dominguez, Y.; Boedi, S.; Sulyok, M.; Wiesenberger, G.; Stoppacher, N.; Krska, R.; Strauss, J. Heterochromatin influences the 866 secondary metabolite profile in the plant pathogen Fusarium graminearum. Fungal Genet. Biol. 2012, 49, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Bueno, J.G.; Martinez, C.; Zapata, B.; Sanclemente, G.; Gallego, M.; Mesa, A.C. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis. Clin. Exp. Dermatol. 2010, 35, 658–663. [Google Scholar] [CrossRef]



| Compound | LogP | Compounds | LogP | Compounds | LogP |
|---|---|---|---|---|---|
| 1 | 1.15 | 6 | 2.37 | 11 | 4.51 |
| 2 | 1.42 | 7 | 3.04 | 12 | 4.78 |
| 3 | 1.54 | 8 | 2.11 | 13 | 3.04 |
| 4 | 2.50 | 9 | 4.44 | ||
| 5 | 2.11 | 10 | 4.90 |
| Species/Species Complex/Sequence Type (ST) | NRRL n.a | PVS-Fu n.b | Diagnosis | Isolate Source | Date |
|---|---|---|---|---|---|
| F. oxysporum/FOSC/ST33 | 46603 | 89 | Onychomycosis | Toe | 2004 |
| F. oxysporum/FOSC/ST33 | 46606 | 91 | Onychomycosis | Toe | 2005 |
| F. keratoplasticum/FSSC/ST2bb | 46443 | 93 | Dermatomycoses | Foot | 2004 |
| F. solani/FSSC/ST5aa | 44903 | 96 | Onychomycosis | Toe | 2006 |
| F. verticillioides/FFSC | 46599 | 87 | Onychomycosis | Toe | 2007 |
| F. verticillioides/FFSC | 46442 | 115 | Onychomycosis | Toe | 2005 |
| Species/Species Complex/Sequence Type (ST) | a PVS-Fu n. | Ester 13 | b TRB | c AmB | |||
|---|---|---|---|---|---|---|---|
| d MIC (µM) | e LD50 (µM) | MIC (µM) | LD50 (µM) | MIC (µM) | LD50 (µM) | ||
| F. oxysporum/FOSC/(ST33) | 89 | >125–250 | 31–62 | >256 | 8–16 | >135 | 16.8–33.7 |
| F. oxysporum/FOSC/(ST33) | 91 | >125–250 | 62–125 | >256 | 16–64 | >135 | 33.7–67.5 |
| F. keratoplasticum/FSSC/(ST2bb) | 93 | 62 | <7.8 | 128–256 | 2.0–4.0 | 33.7–67.5 | >2.1–4.2 |
| F. solani/FSSC/(ST5aa) | 96 | 62–125 | <7.8 | 64–128 | 2.0–4.0 | 4.2–2.1 | 1.0 |
| F. verticillioides/FFSC | 87 | 500 | 62–125 | >256 | 2.0–4.0 | >135 | 2.1–4.2 |
| F. verticillioides/FFSC | 115 | 125–250 | <7.8 | >256 | 2.0–4.0 | >135 | 8.4–16.8 |
| Species | Control | Ester 13 | TRB | AmB |
|---|---|---|---|---|
| 0 (µM) | MIC (250 µM) | MIC (256 µM) | MIC (135 µM) | |
| F. keratoplasticum |  |  |  |  |
 |  |  |  | |
| F. solani |  |  |  |  |
 |  |  |  | |
| F. oxysporum |  |  |  |  |
 |  |  |  | |
| F. verticillioides |  |  |  |  |
 |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oufensou, S.; Casalini, S.; Balmas, V.; Carta, P.; Chtioui, W.; Dettori, M.A.; Fabbri, D.; Migheli, Q.; Delogu, G. Prenylated Trans-Cinnamic Esters and Ethers against Clinical Fusarium spp.: Repositioning of Natural Compounds in Antimicrobial Discovery. Molecules 2021, 26, 658. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030658
Oufensou S, Casalini S, Balmas V, Carta P, Chtioui W, Dettori MA, Fabbri D, Migheli Q, Delogu G. Prenylated Trans-Cinnamic Esters and Ethers against Clinical Fusarium spp.: Repositioning of Natural Compounds in Antimicrobial Discovery. Molecules. 2021; 26(3):658. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030658
Chicago/Turabian StyleOufensou, Safa, Stefano Casalini, Virgilio Balmas, Paola Carta, Wiem Chtioui, Maria A. Dettori, Davide Fabbri, Quirico Migheli, and Giovanna Delogu. 2021. "Prenylated Trans-Cinnamic Esters and Ethers against Clinical Fusarium spp.: Repositioning of Natural Compounds in Antimicrobial Discovery" Molecules 26, no. 3: 658. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030658