Metal Organic Frameworks Derived Fe-N-C Nanostructures as High-Performance Electrodes for Sodium Ion Batteries and Electromagnetic Interference (EMI) Shielding
Abstract
:1. Introduction
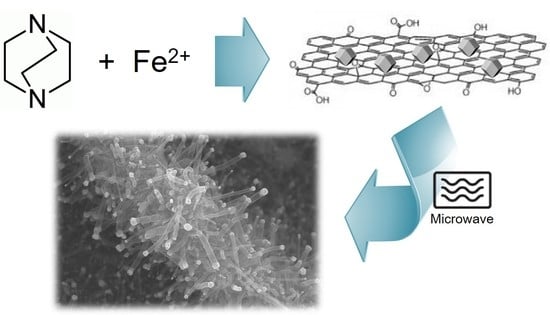
2. Results and Discussion
3. Experimental
Synthesis of 3DCNS from MOF Precursors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Basu, S.; Zeller, C.; Flanders, P.J.; Fuerst, C.D.; Johnson, W.D.; Fischer, J.E. Synthesis and properties of lithium-graphite intercalation compounds. Mater. Sci. Eng. 1979, 38, 275–283. [Google Scholar] [CrossRef]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.-H.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar]
- Araham, K. Intercalation positive electrodes for rechargeable sodium cells. Solid State Ion. 1982, 7, 199–212. [Google Scholar]
- Fan, S.S.; Liu, H.P.; Liu, Q.; Ma, C.S.; Yi, T.F. Comprehensive insights and perspectives into the recent progress of electrode materials for non-aqueous K-ion battery. J. Mater. 2020, 6, 431–454. [Google Scholar] [CrossRef]
- Cahen, S.; Lagrange, P.; Marêché, J.F.; Hérold, C. Analogies and differences between calcium-based and europium-based graphite intercalation compounds. C. R. Chim. 2013, 16, 385–390. [Google Scholar] [CrossRef]
- Nakajima, T.; Kawaguchi, M.; Watanabe, N. Graphite intercalation compound of magnesium fluoride and fluorine. Carbon 1982, 20, 287–291. [Google Scholar] [CrossRef]
- Eisenman, G.; Rudin, D.O.; Casby, J.U. Glass Electrode for Measuring Sodium Ion. Science 1982, 126, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Bandosz, T.J. Building MOF Nanocomposites with Oxidized Graphitic Carbon Nitride Nanospheres: The Effect of Framework Geometry on the Structural Heterogeneity. Molecules 2019, 24, 4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Zhang, P.; Li, K.; Kumari, B.; Li, D.; Mei, X. Silver Nanoclusters Encapsulated into Metal–Organic Frameworks for Rapid Removal of Heavy Metal Ions from Water. Molecules 2019, 24, 2442. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Metal Organic Frameworks Based Materials for Heterogeneous Photocatalysis. Molecules 2018, 23, 2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of Metal-Organic Frameworks in Food Sample Preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, H.; Li, C.; Li, Z. Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater. Molecules 2020, 25, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Meng, X.-G.; Wu, Y.-Y.; Huang, H.; Lv, J.; Yu, W.-W. A Highly Efficient Heterogeneous Catalyst of Bimetal-Organic Frameworks for the Epoxidation of Olefin with H2O2. Molecules 2020, 25, 2389. [Google Scholar] [CrossRef]
- Pérez-Cejuela, H.M.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules 2020, 25, 4216. [Google Scholar] [CrossRef]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Park, H. Zeolitic imidazolate frameworks as novel precursors for microwave synthesis of carbon nanotubes. J. Alloy. Compd. 2019, 781, 166–173. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Microwave induced transformation of metal organic frameworks into defect rich carbon nanofibers. N. J. Chem. 2020, 44, 5666–5672. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, H.; Chen, F.; Liu, X.; Ma, R.; Sasaki, T. Two-dimensional porous cuprous oxide nanoplatelets derived from metal–organic frameworks (MOFs) for efficient photocatalytic dye degradation under visible light. Dalton Trans. 2018, 47, 7694–7700. [Google Scholar] [CrossRef] [PubMed]
- Bita, B. 1, 4-Diazabicyclo [2.2.2] octane (DABCO) as a useful catalyst in organic synthesis. Eur. J. Chem. 2010, 1, 54–60. [Google Scholar] [CrossRef] [Green Version]
- O’Byrne, J.P.; Li, Z.; Jones, S.L.T.; Fleming, P.G.; Larsson, J.A.; Morris, M.A.; Holmes, J.D. Nitrogen-Doped Carbon Nanotubes: Growth, Mechanism and Structure. ChemPhysChem 2011, 12, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Jung, K.H.; Park, H. Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Materials 2020, 13, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, V.; Park, H. Hollow SnO2@carbon core–shell spheres stabilized on reduced graphene oxide for high-performance sodium-ion batteries. N. J. Chem. 2017, 41, 442–446. [Google Scholar] [CrossRef]
- Saito, R.; Tatsumi, Y.; Huang, S.; Ling, X.; Dresselhaus, M.S. Raman spectroscopy of transition metal dichalcogenides. J. Phys. Condens. Matter. 2016, 28, 353002. [Google Scholar] [CrossRef]
- Andresa, J.S.; Moreira, L.M.; Magalhães, J.L.; Gonzalez, E.P.; Landers, R.; Rodrigues-Filho, U.P. XPS analysis of electronic density of iron tetraazamacrocycle through Fe 2p binding energies on the 3-imidazolilpropyl-modified surface of oxidized n-Si(100). Surf. Interface Anal. 2004, 36, 1214–1217. [Google Scholar] [CrossRef]
- Xi, J.; Xia, Y.; Xu, Y.; Xiao, J.; Wang, S. (Fe,Co)@nitrogen-doped graphitic carbon nanocubes derived from polydopamine-encapsulated metal–organic frameworks as a highly stable and selective non-precious oxygen reduction electrocatalyst. Chem. Commun. 2015, 51, 10479–10482. [Google Scholar] [CrossRef]
- Xin, H.; He, D.; Wang, Y.; Zhao, W.; Du, X. Low-temperature preparation of macroscopic nitrogen-doped graphene hydrogel for high-performance ultrafast supercapacitors. RSC Adv. 2015, 5, 8044–8049. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Ma, J.; Yang, X.; Yuan, R.; Chai, Y. Porous Fe2O3-C microcubes as anodes for lithium-ion batteries by rational introduction of Ag nanoparticles. J. Alloy. Comp. 2018, 735, 840–846. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, J.; Chou, S.-L.; Liu, H.K.; Zhang, W.; Zhao, D.; Dou, S.X. Uniform yolk-shell iron sulfide–carbon nanospheres for superior sodium–iron sulfide batteries. Nat. Commun. 2015, 6, 12122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galinato, M.G.I.; Whaley, C.M.; Lehnert, N. Vibrational Analysis of the Model Complex (μ-edt)[Fe(CO)3]2 and Comparison to Iron-Only Hydrogenase: The Activation Scale of Hydrogenase Model Systems Inorg. Inorg. Chem. 2010, 49, 3201–3215. [Google Scholar] [CrossRef] [Green Version]
- Brian, C. Smith in Spectroscopyonline.com, Volume 34, Issue 1, Page Number: 10–15. Available online: https://www.spectroscopyonline.com/view/organic-nitrogen-compounds-part-i-introduction (accessed on 15 February 2021).
- Garai, J.; Haggerty, S.E.; Rekhi, S.; Chance, M. Infrared absorption investigations confirm the extraterrestrial origin of carbonado-diamonds. Astrophys. J. 2006, 653, L153–L156. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, N.; Kahn, M.; Berghauser, R.; Waldhauser, W.; Mizaikoff, B. Nitrogen-Doped Diamond-Like Carbon as Optically Transparent Electrode for Infrared Attenuated Total Reflection Spectroelectrochemistry. Analyst 2011, 136, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lee, J.H.; Hembram, K.P.S.S.; Lee, K.R.; Han, S.S.; Yoon, C.W.; Nam, S.W.; Kim, J.Y. Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon. Catalysts 2016, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Vadahanambi, S.; Chun, H.H.; Jung, K.H.; Park, H. Nitrogen doped holey carbon nanosheets as anodes in sodium ion battery. RSC Adv. 2015, 6, 38112–38116. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Lu, W.; Wang, C.; Xue, W.; Yang, F.; Zhou, J.; Suo, L.; Lin, T.; et al. Nitrogen-Doped Carbon for Sodium-Ion Battery Anode by Self-Etching and Graphitization of Bimetallic MOF-Based Composite. Chem 2017, 3, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kim, D.Y.; Chae, S.A.; Saito, N.; Choi, S.Y.; Kim, K.H. Maximization of sodium storage capacity of pure carbon material used in sodium-ion batteries. J. Mater. Chem. A 2019, 7, 16149–16160. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhang, K.; Yan, F.; Zhu, C.; Yuan, H.; Zhang, X.; Chen, Y. Interface-induced enhanced electromagnetic wave absorption property of metal-organic frameworks wrapped by graphene sheets. J. Alloy. Comp. 2019, 780, 718–726. [Google Scholar] [CrossRef]
- Lü, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zhen, L. MOF-Derived Porous Co/C Nanocomposites with Excellent Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, V.; Lee, I.; Park, H. Metal Organic Frameworks Derived Fe-N-C Nanostructures as High-Performance Electrodes for Sodium Ion Batteries and Electromagnetic Interference (EMI) Shielding. Molecules 2021, 26, 1018. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041018
Sridhar V, Lee I, Park H. Metal Organic Frameworks Derived Fe-N-C Nanostructures as High-Performance Electrodes for Sodium Ion Batteries and Electromagnetic Interference (EMI) Shielding. Molecules. 2021; 26(4):1018. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041018
Chicago/Turabian StyleSridhar, Vadahanambi, Inwon Lee, and Hyun Park. 2021. "Metal Organic Frameworks Derived Fe-N-C Nanostructures as High-Performance Electrodes for Sodium Ion Batteries and Electromagnetic Interference (EMI) Shielding" Molecules 26, no. 4: 1018. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041018