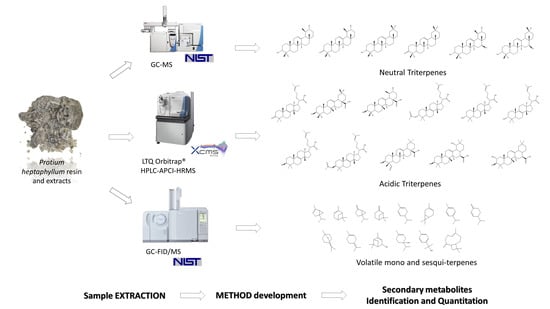
Quali–Quantitative Characterization of Volatile and Non-Volatile Compounds in Protium heptaphyllum (Aubl.) Marchand Resin by GC–MS Validated Method, GC–FID and HPLC–HRMS2
Abstract
:1. Introduction
2. Results
2.1. Volatile Terpenes Identification and Quantification
2.2. Method Validation for Amyrins Quantification
2.3. Quantification of Neutral Triterpens
2.4. Identification and Quantification of Acidic Triterpenes
3. Discussion
3.1. VOC Qualitative and Quantitative Characterization
3.2. Triterpenes Identification and Quantitation
3.2.1. Method Validation for Amyrin Quantification
3.2.2. Neutral Triterpenes Quantitation
3.2.3. Acidic Triterpenic Fraction Identification and Quantification
3.3. Reported Terpenes Biologic Activities
4. Materials and Methods
4.1. Samples of Protium Heptaphyllum
4.2. Chemicals
4.3. Extraction of VOC and Neutral Triterpenes
4.4. Extraction of Acidic Triterpenes
4.5. VOC Profiling and Quantitation
4.6. Neutral Triterpenes GC–MS Detection and Quantification
4.7. Quantitation Method Validation
4.8. Acidic Triterpenic Fraction HPLC–HRMS2 Identification and Quantification
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rüdiger, A.L.; Siani, A.C.; Junior, V.F.V. The chemistry and pharmacology of the South America genus Protium Burm. f.(Burseraceae). Pharmacogn. Rev. 2007, 1, 93–104. [Google Scholar]
- Da Silva, E.R.; De Oliveira, D.R.; De Fátima Figueiredo Melo, M.; Bizzo, H.R.; Leitão, S.G. Report on the Malungo expedition to the Erepecuru river, Oriximiná, Brazil. Part I: Is there a difference between black and white Breu? Braz. J. Pharmacogn. 2016, 26, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomao, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F.; et al. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, P.V.A.; Zapata, F.; Daly, D.C. Investigating processes of neotropical rain forest tree diversification by examining the evolution and historical biogeography of the Protieae (Burseraceae). Evolution 2014, 68, 1988–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerra, J.X.; Venable, D.L.; Evans, P.H.; Bowers, W.S. Interactions Between Chemical and Mechanical Defenses in the Plant Genus Bursera and Their Implications for Herbivores. Am. Zool. 2001, 41, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Aragão, G.F.; Carneiro, L.M.V.; Junior, A.P.F.; Vieira, L.C.; Bandeira, P.N.; Lemos, T.L.G.; Viana, G.D. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol. Biochem. Behav. 2006, 85, 827–834. [Google Scholar] [CrossRef]
- De Lima, E.M.; Cazelli, D.S.P.; Pinto, F.E.; Mazuco, R.A.; Kalil, I.C.; Lenz, D.; Scherer, R.; De Andrade, T.U.; Endringer, D.C. Essential oil from the resin of Protium heptaphyllum: Chemical composition, cytotoxicity, antimicrobial activity, and antimutagenicity. Pharmacogn. Mag. 2016, 12, S42–S46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siani, A.C.; Nakamura, M.J.; Tappin, M.R.R.; Monteiro, S.S.; Guimarães, A.C.; Ramos, M.F.S. Chemical Composition of South American Burseraceae Nonvolatile Oleoresins and Preliminary Solubility Assessment of their Commercial Blend. Phytochem. Anal. 2012, 23, 529–539. [Google Scholar] [CrossRef]
- Bandeira, P.N.; Machado, M.I.L.; Cavalcanti, F.S.; Lemos, T.L.G. Essential Oil Composition of Leaves, Fruits and Resin of Protium heptaphyllum (Aubl.) March. J. Essent. Oil Res. 2001, 13, 33–34. [Google Scholar] [CrossRef]
- Lima, E.M.; Nascimento, A.M.; Lenz, D.; Scherer, R.; Meyrelles, S.S.; Boëchat, G.A.P.; Andrade, T.U.; Endringer, D.C. Triterpenes from the Protium heptaphyllum resin—chemical composition and cytotoxicity. Rev. Bras. De Farmacogn. 2014, 24, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.R.; Zoghbi, M.D.; Pinto, A.D.; Godoy, R.L.; Amaral, A.C. Analysis of the Hexane Extracts From Seven Oleoresins of Protium Species. J. Essent. Oil Res. 2009, 21, 305–308. [Google Scholar] [CrossRef]
- Neves, G.P.; Nakamura, M.J.; Ramos, M.F.; Siani, A.C.; Mazzei, J.L. Development of a gas chromatography method for quantification of triterpenes in the commercial oleoresins from Protium species. Rodriguésia 2020, 71. [Google Scholar] [CrossRef]
- Susunaga, G.S.; Siani, A.C.; Pizzolatti, M.G.; Yunes, R.A.; Delle Monache, F. Triterpenes from the resin of Protium heptaphyllum. Fitoterapia 2001, 72, 709–711. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.C.; Lima, R.C.P.; Silva, R.M.; Maia, J.L.; Brito, G.A.A.C.; Santos, F.A.; Rao, V.S. Protective effect of α- and β-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J. Ethnopharmacol. 2005, 98, 103–108. [Google Scholar] [CrossRef]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragao, G.F. Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: A literature review. Fundam. Clin. Pharmacol. 2019, 33, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 10–54. Available online: https://www.allured.com/ (accessed on 20 December 2020).
- Administration, F.D. Guidance for Industry: Bioanalytical Method Validation. 2001. Available online: http://www.fda.gov/cder/Guidance/4252fnl.pdf (accessed on 20 December 2020).
- Agency, E.M. Guideline on bioanalytical method validation. Eur. Med. Agency 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 20 December 2020).
- Fiehn, O.; Robertson, D.; Griffin, J.; van der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L.W.; Goodacre, R.; Hardy, N.W.; Taylor, C.; et al. The metabolomics standards initiative (MSI). Metabolomics 2007, 3, 175–178. [Google Scholar] [CrossRef]
- Albino, R.C.; Oliveira, P.C.; Prosdocimi, F.; da Silva, O.F.; Bizzo, H.R.; Gama, P.E.; Sakuragui, C.M.; Furtado, C.; de Oliveira, D.R. Oxidation of monoterpenes in Protium heptaphyllum oleoresins. Phytochemistry 2017, 136, 141–146. [Google Scholar] [CrossRef]
- Mendes, J.L.; de Araújo, T.F.; Geraldo de Carvalho, M.; Aragão Catunda Júnior, F.E.; Albuquerque Costa, R. Chemical Composition and Mechanism of Vibriocidal Action of Essential Oil from Resin of Protium heptaphyllum. Sci. World J. 2019, 2019, 9563213. [Google Scholar] [CrossRef] [Green Version]
- Mobin, M.; de Lima, S.G.; Almeida, L.T.G.; Silva Filho, J.C.; Rocha, M.S.; Oliveira, A.P.; Mendes, M.B.; Carvalho, F.A.A.; Melhem, M.S.C.; Costa, J.G.M. Gas Chromatography-Triple Quadrupole Mass Spectrometry Analysis and Vasorelaxant Effect of Essential Oil from Protium heptaphyllum (Aubl.) March. Biomed Res. Int. 2017, 2017, 1928171. [Google Scholar] [CrossRef] [Green Version]
- Siani, A.C.; Ramos, M.F.; Monteiro, S.D.; Ribeiro-dos-Santos, R.; Soares, R.O. Essential oils of the oleoresins from Protium Heptaphyllum growing in the Brazilian Southeastern and their cytotoxicity to neoplasic cells lines. J. Essent. Oil Bear. Plants 2011, 14, 373–378. [Google Scholar] [CrossRef]
- Rhourri-Frih, B.; Chaimbault, P.; Claude, B.; Lamy, C.; André, P.; Lafosse, M. Analysis of pentacyclic triterpenes by LC-MS. A comparative study between APCI and APPI. J. Mass Spectrom. 2009, 44, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.; Lin, H.S.; Tan, C.H.; Koh, H.L. Quantification of α- And β-amyrin in rat plasma by gas chromatography-mass spectrometry: Application to preclinical pharmacokinetic study. J. Mass Spectrom. 2011, 46, 457–464. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A simple GC–MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Kpoviéssi, D.S.S.; Gbaguidi, F.; Gbénou, J.; Accrombessi, G.; Moudachirou, M.; Rozet, E.; Hubert, P.; Quetin-Leclercq, J. Validation of a method for the determination of sterols and triterpenes in the aerial part of Justicia anselliana (Nees) T. Anders by capillary gas chromatography. J. Pharm. Biomed. Anal. 2008, 48, 1127–1135. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Elfakir, C. Development of a gas chromatography–mass spectrometry method to monitor in a single run, mono- to triterpenoid compounds distribution in resinous plant materials. J. Chromatogr. A 2016, 1443, 241–253. [Google Scholar] [CrossRef]
- Babalola, I.T.; Shode, F.O. Ubiquitous Ursolic Acid: A Potential Pentacyclic Triterpene Natural Product. Pharm. Phytochem 2013, 2, 214–222. [Google Scholar]
- Yousuf, S.; Kamdem, R.S.T.; Ngadjui, B.T.; Wafo, P.; Fun, H.-K. 3α-Hydroxytirucalla-8,24-dien-21-oic acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o937–o938. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.J.; Delgado, G.; Díaz de Delgado, G.; Usubillaga, A.; Khouri, N.; Bahsas, A. 3α-Hydroxytirucalla-7,24-dien-21-oic acid: A triterpene from Protium crenatum Sandwith. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminformatics 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tautenhahn, R.; Cho, K.; Uritboonthai, W.; Zhu, Z.; Patti, G.J.; Siuzdak, G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012, 30, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. Isrn Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Lopes, P.M.; Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of a-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Fragr. J. 2020. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Aly, E.; Khajah, M.A.; Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutierrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Guedes, M.A.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid α,β-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine 2008, 15, 630–634. [Google Scholar] [CrossRef]
- Aragão, G.F.; Cunha Pinheiro, M.C.; Nogueira Bandeira, P.; Gomes Lemos, T.L.; de Barros Viana, G.S. Analgesic and anti-inflammatory activities of the isomeric mixture of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) march. J. Herb. Pharmacother. 2007, 7, 31–47. [Google Scholar]
- Lima, R.C.P.; Oliveira, F.A.; Gurgel, L.A.; Cavalcante, Í.J.M.; Santos, K.A.; Campos, D.A.; Vale, C.A.L.; Silva, R.M.; Chaves, M.H.; Rao, V.S.N.; et al. Attenuation of visceral nociception by α- and β- amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum, in mice. Planta Med. 2006, 72, 34–39. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Vieira-Júnior, G.M.; Chaves, M.H.; Almeida, F.R.C.; Florêncio, M.G.; Lima, R.C.P.; Silva, R.M.; Santos, F.A.; Rao, V.S.N. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol. Res. 2004, 49, 105–111. [Google Scholar] [CrossRef]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Santos, F.A.; Frota, J.T.; Arruda, B.R.; De Melo, T.S.; Da Silva, A.A.D.C.A.; Brito, G.A.D.C.; Chaves, M.H.; Rao, V.S. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Sakurai, Y.; Chen, C.-H.; Chang, F.-R.; Huang, L.; Kashiwada, Y.; Lee, K.-H. Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents. J. Med. Chem. 2006, 49, 5462–5469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, Z.; Lu, C.; Zhao, S.; Wang, J.; Liu, B.; Xu, X.; Liu, Y. Triterpenoid resinous metabolites from the genus Boswellia: Pharmacological activities and potential species-identifying properties. Chem. Cent. J. 2013, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-T.; Lu, C.-L.; Lin, H.-I.; Chen, B.-F.; Jow, G.-M. β-elemonic acid inhibits the cell proliferation of human lung adenocarcinoma A549 cells: The role of MAPK, ROS activation and glutathione depletion. Oncol. Rep. 2016, 35, 227–234. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Zaprutko, L. Anti-COVID drugs: Repurposing existing drugs or search for new complex entities, strategies and perspectives. Future Med. Chem. 2020, 12, 1743–1757. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, V.K.; Egbuna, C.; Tiwari, M. Plant secondary metabolites as lead compounds for the production of potent drugs. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier science; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 3–14. [Google Scholar]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compound Name | LRIC 2 | LRIL 2 | Concentration 1 | |||||
|---|---|---|---|---|---|---|---|---|
| PHR | ATCE | AMCE | ||||||
| α-thujene | 926 | 924 | 0.49 | ±0.08 | 0.19 | ±0.01 | 0.97 | ±0.09 |
| α-pinene | 932 | 932 | 12.64 | ±1.91 | 4.33 | ±0.07 | 9.13 | ±0.71 |
| sabinene | 972 | 969 | 0.74 | ±0.15 | 0.17 | ±0.02 | 1.60 | ±0.22 |
| β-pinene | 976 | 974 | 1.33 | ±0.14 | 0.80 | ±0.01 | 1.03 | ±0.10 |
| α-phellandrene | 1005 | 1002 | 4.21 | ±0.13 | 3.39 | ±0.05 | 9.78 | ±0.71 |
| δ-3-carene | 1008 | 1008 | 7.04 | ±0.005 | 7.34 | ±0.08 | 12.14 | ±1.05 |
| p-cymene | 1023 | 1020 | 11.11 | ±1.33 | 9.36 | ±0.02 | 3.49 | ±0.31 |
| β-phellandrene | 1028 | 1025 | 6.73 | ±1.11 | 4.72 | ±0.03 | 8.87 | ±0.79 |
| 1,8-cineole | 1030 | 1026 | 0.75 | ±0.03 | 1.09 | ±0.04 | 0.12 | ±0.01 |
| α-terpinolene | 1088 | 1086 | 0.50 | ±0.02 | 0.82 | ±0.03 | 0.67 | ±0.01 |
| trans-Verbenol | 1144 | 1140 | 1.29 | ±0.05 | 1.78 | ±0.06 | 0.00 | ±0.00 |
| terpinen-4-ol | 1177 | 1174 | 0.57 | ±0.10 | 0.84 | ±0.01 | 0.23 | ±0.03 |
| p-cymen-8-ol | 1184 | 1179 | 1.19 | ±0.02 | 1.41 | ±0.01 | 0.00 | ±0.00 |
| β-e-caryophyllene | 1422 | 1417 | 2.15 | ±0.09 | 4.58 | ±0.07 | 0.69 | ±0.20 |
| sum | 50.75 | ±2.60 | 40.28 | ±0.44 | 48.74 | ±4.21 | ||
| Parameter | α-Amyrin | β-Amyrin | Acceptability Range | ||||
|---|---|---|---|---|---|---|---|
| R2 | 0.9965 (0.9966) | 0.9977 (0.9990) | 0.9966 (0.9994) | 0.9994 (0.9995) | 0.9963 (0.9990) | 0.9961 (0.9995) | ≥0.995 |
| DIFF% | 11.55 (7.15) | 3.33 (6.49) | 14.89 (13.63) | 5.67 (1.52) | 10.06 (13.63) | 15.73 (15.16) | ≤25 |
| SEL% | 7.01 | 7.80 | ≤30 | ||||
| LOD (mg/L) | 0.20 (0.11) | 0.24 (0.07) | |||||
| LOQ (mg/L) | 0.67 (0.39) | 0.79 (0.25) | |||||
| RSD% 0.5 mg/L (0.1 mg L−1) | 18.87 (16.95) | 16.81 (21.94) | ≤25 | ||||
| ERR% 0.5 mg L−1 (0.1 mg L−1) | 13.62 (18.3) | 4.66 (25.6) | 14.95 (24.6) | 13 (12.4) | 19.10 (18.4) | 8.38 (15.9) | ≤20 |
| Compound | RT (min) | Quantitative Ion (m/z) | Concentration (g kg−1) ± SD 1 | ||
|---|---|---|---|---|---|
| PHR (n = 3) | ATCE (n = 4) | AMCE (n = 3) | |||
| α-amyrin | 15.77 | 218 | 251.28 ± 19.34 | 123.98 ±13.92 | 556.82 ± 30.49 |
| β-amyrin | 15.42 | 218 | 172.66 ± 21.42 | 95.39 ± 11.66 | 385.58 ± 21.82 |
| α-amyron | 15.59 | 218 | 18.41 ± 0.86 | 21.71 ± 0.81 | n.d. 2 |
| β-amyron | 15.28 | 218 | 14.31 ± 0.61 | 12.92 ± 6.18 | n.d. 2 |
| Maniladiol | 17.53 | 234 | 12.20 ± 1.18 | 9.36 ± 0.68 | 17.00 ± 0.90 |
| Brein | 18.04 | 234 | 14.92 ± 2.27 | 12.87 ± 0.82 | 20.88 ± 0.67 |
| Sum (g kg−1) | 483.77 ± 43.23 | 270.68 ± 22.75 | 980.28 ± 51.39 | ||
| ID# | Putative Name | Formula | MSI Level | Rt (min) | m/z [M-H]− | MS/MS (m/z) | Concentration (g kg−1) ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHR n = 3 | sd | ATCE n = 3 | sd | AMCE n = 3 | sd | |||||||
| 1 | 3-oxo -tirucalla-8,24-dien-21-oic acid | C30H46O3 | 1 | 15.1 | 453.3384 | 435.3234, 371.2563 339.2671 | 80.64 | ±3.23 | 157.10 | ±0.99 | 15.31 | ±0.38 |
| 2,3 | Oleanolic-Ursolic acid | C30H48O3 | 1 | 12.3 | 455.3510 | 407.3306 | 1.85 | ±0.52 | 2.40 | ±0.13 | 1.93 | ±0.03 |
| 4 | 3α-Hydroxy-tirucalla-7,24-dien-21-oic acid | C30H48O3 | 1 | 14.3 | 455.3511 | 437.3391, 373.2720, 339.2676 | 7.62 | ±0.18 | 3.77 | ±1.21 | 1.26 | ±0.45 |
| 5 | 3α-Hydroxy-tirucalla-8,24-dien-21-oic acid | C30H48O3 | 1 | 14.9 | 455.3541 | 437.3394, 373.2720, 373.2728 | 77.71 | ±1.29 | 130.40 | ±13.21 | 11.64 | ±4.24 |
| 6 | 3β-Hydroxy-tirucalla-8,24-dien-21-oic acid | C30H48O3 | 1 | 15.9 | 455.3540 | 437.3391, 409.3411, 373.2720 | 60.14 | ±9.66 | 107.87 | ±7.13 | 8.38 | ±2.37 |
| 7 | Gypsogenin | C30H46O4 | 2 | 10.4 | 469.3324 | 451.3125, 391.2664, 358.2769 | 0.34 | ±0.02 | 0.35 | ±0.03 | n.d. | n.d. |
| 8 | Siaresinol | C30H48O4 | 2 | 13.7 | 471.3490 | 453.3341, 389.2664, 357.2771 | 2.99 | ±0.15 | 3.73 | ±0.43 | n.d. | n.d. |
| 9 | Maslinic Acid | C30H48O4 | 2 | 14.6 | 471.3488 | 453.3344, 389.2660, 357.2771 | 2.90 | ±0.07 | 4.07 | ±0.58 | n.d. | n.d. |
| 10 | 3α Acetyl -tirucalla-8,24-dien-21-oic acid | C32H50O4 | 2 | 17.1 | 497.3641 | 437.3434, 479.3541, 415.2862 | 4.01 | ±1.25 | 6.29 | ±0.66 | n.d. | n.d. |
| 11 | 3β-Acetyl-tirucalla-8,24-dien-21-oic acid | C32H50O4 | 2 | 18.0 | 497.3640 | 437.3431, 479.3544, 415.2862 | 6.62 | ±2.47 | 12.71 | ±2.03 | n.d. | n.d. |
| Sum (g kg−1) | 234.65 | ±14.39 | 415.26 | ±18.98 | 30.23 | ±6.06 | ||||||
| Sample Name | Batch | Description |
|---|---|---|
| PHR | A0000151 | Protium heptaphyllum (Aubl.) Marchand oleum resin raw material |
| ATCE | P75-2-0 | Acidic Triterpenes Concentrated Extract obtained from PHR sample |
| AMCE | P75-2-1 | A- and β-Amyrins Concentrated Extract obtained from PHR sample |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asteggiano, A.; Occhipinti, A.; Capuzzo, A.; Mecarelli, E.; Aigotti, R.; Medana, C. Quali–Quantitative Characterization of Volatile and Non-Volatile Compounds in Protium heptaphyllum (Aubl.) Marchand Resin by GC–MS Validated Method, GC–FID and HPLC–HRMS2. Molecules 2021, 26, 1447. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26051447
Asteggiano A, Occhipinti A, Capuzzo A, Mecarelli E, Aigotti R, Medana C. Quali–Quantitative Characterization of Volatile and Non-Volatile Compounds in Protium heptaphyllum (Aubl.) Marchand Resin by GC–MS Validated Method, GC–FID and HPLC–HRMS2. Molecules. 2021; 26(5):1447. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26051447
Chicago/Turabian StyleAsteggiano, Alberto, Andrea Occhipinti, Andrea Capuzzo, Enrica Mecarelli, Riccardo Aigotti, and Claudio Medana. 2021. "Quali–Quantitative Characterization of Volatile and Non-Volatile Compounds in Protium heptaphyllum (Aubl.) Marchand Resin by GC–MS Validated Method, GC–FID and HPLC–HRMS2" Molecules 26, no. 5: 1447. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26051447