Using Micropropagation to Develop Medicinal Plants into Crops
Abstract
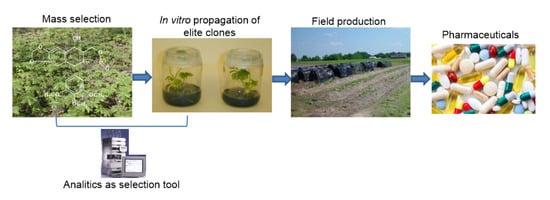
:1. Introduction
2. Pharmaceutical Crops for Production of Small Therapeutic Molecules (STM)
3. Phytomedicine Crops for the Production of Standardized Therapeutic Natural Products
4. Micropropagation of Plants for Production of Large Therapeutic Molecules (LTM) Including Fructooligosaccharides Classified as Prebiotic
5. Functional Food Crops
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant in vitro Culture; Leva, A., Rinaldi, L.M.R.L., Eds.; InTech: London, UK, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Chung, S.M.; Vaidya, M.; Tzfira, T. Agrobacterium is not alone: Gene transfer to plants by viruses and other bacteria. Trends Plant Sci. 2006, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, B.; Haddad, R.; Bandehpour, M. Efficient plant regeneration and Agrobacterium-mediated transformation via somatic embryogenesis in purslane (Portulaca oleracea L.): An important medicinal plant. Plant Cell Tissue Organ Cult. 2019, 136, 231–245. [Google Scholar] [CrossRef]
- Mohan, N.; Nikdad, S.; Singh, G. Studies on seed germination and embryo culture of Jatropha curcas L. under in vitro conditions. Biotech. Bioinf. Bioenergy 2011, 1, 187–194. [Google Scholar]
- Moraes-Cerdeira, R.M.; Burandt, C.L.; Bastos, J.K.; Nanayakkara, N.P.D.; McChesney, J.D. In vitro propagation of Podophyllum peltatum. Planta Med. 1998, 64, 42–45. [Google Scholar] [CrossRef]
- Mestinsek-Mubi, S.; Svetik, S.; Flajsman, M.; Murovec, J. In vitro tissue culture and genetic analysis of two high-CBD medical cannabis (Cannabis sativa L.) breeding lines. Genetika 2020, 52, 925–941. [Google Scholar] [CrossRef]
- Lata, H.; Moraes, R.M.; Bertoni, B.; Pereira, A.M.S. In vitro germplasm conservation of Podophyllum peltatum L. under slow growth conditions. In vitro Cell. Dev. Biol. Plant 2010, 46, 22–27. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic Homogeneity Revealed Using SCoT, ISSR and RAPD Markers in Micropropagated Pittosporum eriocarpum Royle- An Endemic and Endangered Medicinal Plant. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- França, S.; Duarte, I.; Moraes, R.; Pereira, A.M.S. Micropropagation of Stryphnodendron polyphythum (Barbatimão). Plant Cell Tissue Organ Cult. 1995, 42, 291–293. [Google Scholar] [CrossRef]
- Patel, A.K.; Lodha, D.; Shekhawat, N.S. An improved micropropagation protocol for the ex situ conservation of Mitragyna parvifolia (Roxb.) Korth. (Rubiaceae): An endangered tree of pharmaceutical importance. In vitro Cell. Dev. Biol. Plant 2020, 56, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef]
- Halder, M.; Roychowdhury, D.; Jha, S. A Critical Review on Biotechnological Interventions for Production and Yield Enhancement of Secondary Metabolites in Hairy Root Cultures. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018; pp. 21–44. [Google Scholar]
- Lata, H.; Li, X.C.; Silva, B.; Moraes, R.M.; Halda-Alija, L. Identification of IAA-producing endophytic bacteria from micropropagated Echinacea plants using 16S rRNA sequencing. Plant Cell Tissue Organ Cult. 2006, 85, 353–359. [Google Scholar] [CrossRef]
- Rosa, L.; Tabanca, N.; Techen, N.; Wedge, D.; Pan, Z.; Bernier, U.; Becnel, J.; Agramonte, N.; Walker, L.; Moraes, R. Diversity and Biological Activities of Endophytic Fungi Associated with Micropropagated Medicinal Plant Echinacea purpurea (L.) Moench. Am. J. Plant Sci. 2012, 3, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.; Lata, H.; Sumyanto, J.; Pereira, A.; Bertoni, B.; Joshi, V.; Pugh, N.; Khan, I.; Pasco, D. Characterization and pharmacological properties of in vitro propagated clones of Echinacea tennesseensis (Beadle) Small. Plant Cell Tissue Organ Cult. 2011, 106, 309–315. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Yang, P.; Antoun, M.; Balick, M.; Cragg, G. Pharmaceutical crops: An overview. Pharm. Crop 2010, 1, 1–17. [Google Scholar] [CrossRef]
- Cragg, G.; Newman, D. Plants as a source of anti-cancer and anti-HIV agents. Ann. Appl. Biol. 2003, 143, 127–133. [Google Scholar] [CrossRef]
- McChesney, J.; Venkataraman, S.; Henri, J. Plant natural products: Back to the future or into extinction? Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Foster, S. Medicinal plant conservation and genetic resources: Examples from the temperate Northern hemisphere. Acta Hortic. 1993, 330, 67–74. [Google Scholar] [CrossRef]
- Cragg, G.; Schepartz, S.; Suffness, M.; Grever, M. The Taxol Supply Crisis. New NCI Policies for Handling the Large-Scale Production of Novel Natural Product Anticancer and Anti-HIV Agents. J. Nat. Prod. 1993, 56, 1657–1668. [Google Scholar] [CrossRef]
- Bastos, J.K.; Burandt, C.L.; Nanayakkara, N.P.D.; Bryant, L.; McChesney, J.D. Quantitation of Aryltetralin Lignans in Plant Parts and among Different Populations of Podophyllum peltatum by Reversed-Phase High-Performance Liquid Chromatography. J. Nat. Prod. 1996, 59, 406–408. [Google Scholar] [CrossRef]
- Bedir, E.; Khan, I.; Moraes, M.R. Bioprospecting for podophyllotoxin. In Trends in New Crops and New uses; Janik, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2001; pp. 545–549. [Google Scholar]
- Elsohly, H.; Croom, E.; Kopycki, W.; Joshi, A.; Elsohly, M.; McChesney, J. Concentrations of taxol and related taxanes in the needles of different Taxus cultivars. Phytochem. Anal. 1995, 6, 149–156. [Google Scholar] [CrossRef]
- Sisti, N.J.; Brinkman, H.R.; McChesney, J.D.; Chander, M.D.; Liang, X.; Zygmunt, J. Methods and useful intermediates for paclitaxel synthesis from C-7, C-10 di-cbz 10-deacetylbaccatin III. Patent US 6448417B1, 10 September 2002. [Google Scholar]
- Majada, J.; Sierra, M.; Sánchez-Tamés, R. One step more towards taxane production through enhanced Taxus propagation. Plant Cell Rep. 2000, 19, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Canel, C.; Dayan, F.; Ganzera, M.; Khan, I.; Rimando, A.; Burandt, C.; Moraes, R. High Yield of Podophyllotoxin from Leaves of Podophyllum peltatum by In situ Conversion of Podophyllotoxin 4-O-β-D-Glucopyranoside. Planta Med. 2001, 67, 97–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, R.; Burandt, C.; Ganzera, M.; Li, X.; Khan, I.; Canel, C. The American mayapple revisited—Podophyllum peltatum—Still a potential cash crop? Econ. Bot. 2000, 54, 471–476. [Google Scholar] [CrossRef]
- Moraes, R.; Momm, H.; Silva, B.; Maddox, V.; Easson, G.; Lata, H.; Ferreira, D. Geographic Information System Method for Assessing Chemo-Diversity in Medicinal Plants. Planta Med. 2006, 71, 1157–1164. [Google Scholar] [CrossRef]
- Graham, I.; Besser, K.; Blumer, S.; Branigan, C.; Czechowski, T.; Elias, L.; Guterman, I.; Harvey, D.; Isaac, P.; Khan, A.; et al. The Genetic Map of Artemisia annua L. Identifies Loci Affecting Yield of the Antimalarial Drug Artemisinin. Science 2010, 327, 328–331. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Janick, J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tissue Organ Cult. 1996, 44, 211–217. [Google Scholar] [CrossRef]
- Wetzstein, H.; Porter, J.; Janick, J.; Ferreira, J.F.S.; Mutui, T.M. Selection and Clonal Propagation of High Artemisinin Genotypes of Artemisia annua. Front Plant Sci. 2018, 9, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne, H.; Berthoulym, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, K.; Sinha, R.; Kumar, N. In Vitro Plant Propagation of Catharanthus roseus and Assessment of Genetic Fidelity of Micropropagated Plants by RAPD Marker Assay. Appl. Biochem. Biotechnol. 2013, 169, 804–900. [Google Scholar] [CrossRef] [PubMed]
- Nacheva, L.; Dimitrova, N.; Ivanova, V.; Cao, F.; Zhu, Z. Micropropagation of Camptotheca Acuminata Decne (Nyssaceae)—Endangered Ornamental and Medicinal Tree. Acta Univ. Agric. Silvic. 2020, 68, 679–686. [Google Scholar]
- Zagorska, N.; Stanilova, M.; Ilcheva, V.; Gadeva, P. Micropropagation of Leucojum aestivum L. (Summer Snowflake). In High-Tech and Micropropagation VI; Bajaj, Y.P.S., Ed.; Springer: Heidelberg, Germany, 1997; Volume 40, pp. 178–192. [Google Scholar] [CrossRef]
- Khonakdari, M.; Rezadoost, H.; Heydari, R.; Mirjalili, M. Effect of photoperiod and plant growth regulators on in vitro mass bulblet proliferation of Narcissus tazzeta L. (Amaryllidaceae), a potential source of galantamine. Plant Cell Tissue Organ Cult. 2020, 142, 187–199. [Google Scholar] [CrossRef]
- Uranbey, S. Thidiazuron induced adventitious shoot regeneration in Hyoscyamus niger. Biol. Plantarum. 2005, 49, 427–430. [Google Scholar] [CrossRef]
- Sabá, R.; Lameira, O.; Luz, J.; Gomes, A.; Innecco, R. Micropropagation of the jaborandi. Hortic. Bras. 2002, 20, 106–109. [Google Scholar]
- Chakraborty, A.; Bhattacharya, D.; Ghanta, S.; Chattopadhyay, S. An efficient protocol for in vitro regeneration of Podophyllum hexandrum, a critically endangered medicinal plant. Indian J. Biotech. 2010, 9, 217–220. [Google Scholar]
- Bhatt, R.; Arif, M.; Gaur, A.K.; Rao, P.B. Rauwolfia serpentina: Protocol optimization for in vitro propagation. Afr. J. Biotechnol. 2009, 7, 4265–4268. [Google Scholar]
- Abbasin, Z.; Zamani, S.; Movahedi, S.; Khaksar, G.; Tabatabaei, B.E.S. In vitro micropropagation of yew (Taxus baccata) and Production of Plantlets. Biotechnology 2010, 9, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Govidaraghavan, S.; Sucher, N.J. Quality Assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicine. Epilepsy Behav. 2015, 52, 363–371. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Memento Fitoterápico da Farmacopéia Brasileira; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2016. [Google Scholar]
- Canter, P.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tamta, H.; Pugh, N.D.; Balachandran, P.; Moraes, R.; Sumiyanto, J.; Pasco, D. Variability of In Vitro Macrophage Activation by Commercially Diverse Bulk Echinacea Plant Material is Due Predominantly to Bacterial Lipoproteins and Lipopolysaccharides. J. Agr. Food Chem. 12009, 56, 10552–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhard, K.H. Uncaria tomentosa (Willd) D.D. Cat’s Claw, Una de Gato or Saventraro. J. Altern. Complement. Med. 1999, 5, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Morais, S.R.; Oliveira, T.L.S.; Oliveira, L.P.; Tresvenzol, L.M.F.; da Conceicao, E.C.; Rezende, M.H.; Fiuza, T.d.S.; Costa, E.A.; Ferri, P.H.; de Paula, J.R. Essential Oil Composition, Antimicrobial and Pharmacological Activities of Lippia sidoides Cham. (Verbenaceae) From São Gonçalo do Abaeté, Minas Gerais. Brazil. Pharmacogn. Mag. 2016, 12, 262–270. [Google Scholar]
- Cavalcanti, S.; Niculau, E.S.; Blank, A.; Camara, C.; Araújo, I.N.; Alves, P. Composition and acaricidal activity of Lippia sidoides essential oil against two-spotted spider mite (Tetranychus urticae Koch). Bioresour. Technol. 2009, 101, 829–832. [Google Scholar] [CrossRef]
- Marco, C.; Teixeira, E.; Simplicio, A.A.F.; Oliveira, C.; Costa, J.; Feitosa, J. Chemical composition and allelopathyc activity of essential oil of Lippia sidoides Cham. Chil. J. Agr. Res. 2012, 72, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.; Cardoso, M.; Moraes, J.C.; Carvalho, S.; Rodrigues, V.; Guimarães, L. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham. and monoterpenes against Tenebrio molitor (L.) (Coleoptera: Tenebrionidae). Cienc. Agrotec. 2011, 35, 664–671. [Google Scholar] [CrossRef]
- Santos, C.P.; Oliveira, T.C.; Pinto, J.A.O.; Fontes, S.S.; Cruz, E.M.O.; Arrigoni-Blank, M.F.; Andrade, T.M.A.; Matos, I.L.; Alves, P.B.; Innecco, R.; et al. Chemical diversity and influence of plant age on the essential oil from Lippia sidoides Cham. Germplasm. Ind. Crop. Prod. 2015, 76, 416–421. [Google Scholar] [CrossRef]
- Costa, A.; Arrigoni-Blank, M.; Blank, A.; Mendonça, A.; Amancio, V.; Lédo, A. In vitro establishment of Lippia sidoides Cham. Hortic. Bras. 2007, 25, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Souza-Moreira, T.; Queiroz-Fernandes, G.; Pietro, R. Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules 2018, 23, 910. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, V.; Cerdeira, A.; Fachin, A.; Bertoni, B.; Pereira, P.; França, S.; Momm, H.; Moraes, R.; Pereira, A. Geographical variation and quality assessment of Stryphnodendron adstringens (Mart.) Coville within Brazil. Genet. Resour. Crop. Evol. 2012, 59, 1349–1356. [Google Scholar] [CrossRef]
- Lata, H.; Bedir, E.; Hosick, A.; Ganzera, M.; Khan, I.; Moraes, R. In vitro Plant Regeneration from Leaf-Derived Callus of Cimicifuga racemosa. Planta Med. 2002, 68, 912–915. [Google Scholar] [CrossRef]
- Sedivá, J.; Vlašínová, H.; Mertelík, J. Shoot regeneration from various explants of horse chestnut (Aesculus hippocastanum L.). Sci. Hortic. 2013, 161, 223–227. [Google Scholar] [CrossRef]
- Ayabe, M.; Sumi, S. Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Rep. 1998, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sarkar, A. In vitro regeneration and micropropagation of Aloe vera L. Sci. Hortic. 1991, 47, 107–113. [Google Scholar] [CrossRef]
- Çöçü, S.; Uranbey, S.; İpek, A.; Khawar, K.M.; Sarihan, E.O.; Kaya, M.D.; Parmaksız, I.; Özcan, S. Adventitious Shoot Regeneration and Micropropagation in Calendula officinalis L. Biol. Plant. 2004, 48, 449–451. [Google Scholar] [CrossRef]
- El Boullani, R.; Elmoslih, A.; Elfinti, A.; Abdelhamid, E.M.; Serghini, M. Improved in Vitro Micropropagation of Artichoke (Cynara cardunculus var. scolymus L.). Eur. J. Sci. Res. 2012, 80, 430–436. [Google Scholar]
- Jones, A.; Yi, Z.; Murch, S.; Saxena, P. Thidiazuron-induced regeneration of Echinacea purpurea L.: Micropropagation in solid and liquid culture systems. Plant Cell Rep. 2007, 26, 13–19. [Google Scholar] [CrossRef]
- Camper, N.; Coker, P.; Wedge, D.; Keese, R. In vitro culture of Ginkgo. In Vitro Cell. Dev. Biol. Plant 1997, 33, 125–127. [Google Scholar] [CrossRef]
- Kaliamoorthy, S.; Naidoa, G.; Achar, P. Micropropagation of Harpagophytum procumbens. Biol. Plantarum 2008, 52, 191–194. [Google Scholar] [CrossRef]
- Gadzovska Simic, S.; Maury, S.; Saida, O.; Righezza, M.; Kascakova, S.; Refregiers, M.; Spasenoski, M.; Joseph, C.; Hagège, D. Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol. Biochem. 2005, 591–601. [Google Scholar] [CrossRef]
- Taniguchi, H.; Takano, H.T. Micropropagation of Matricaria chamomilla (Camomile). In High-Tech and Micropropagation VI; Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 332–342. [Google Scholar]
- Pereira, A.; Moro, J.; Cerdeira, R.; França, S. Effect of phytoregulators and physiological characteristics of the explants on micropropagation of Maytenus ilicifolia. Plant Cell Tissue Organ Cult. 1995, 42, 295–297. [Google Scholar] [CrossRef]
- Ożarowski, M.; Thiem, B. Progress in micropropagation of Passiflora spp. to produce medicinal plants: A mini-review. Rev. Bras. Farmacogn. 2013, 23, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, C.B.; Mendes, L.A.C. Estabelecimento de protocolo para o cultivo in vitro do guarana (Paullinia cupana (Mart.) Ducke). In Anais da 1 Jornada de Iniciação Científica da Embrapa Amazonia; Embrapa: Brasilia, Brazil, 2004. [Google Scholar]
- Rios, D.; Sandoval, D.; Gomes, C. In vitro culture of Peumus boldus Molina via direct organogenesis. J. Med. Chem. 2010, 21, 70–72. [Google Scholar]
- Zhang, Z.; Zhao, L.; Chen, X.; Zheng, X. Sucessful micropropagation protocol of Piper methysticum. Biol. Plantarum. 2008, 52, 110–112. [Google Scholar] [CrossRef]
- Rawls, B.; Harris-Shultz, K.; Dhekney, S.; Forrester, I.; Sitther, V. Clonal Fidelity of Micropropagated Psidium guajava L. Plants Using Microsatellite Markers. Am. J. Plant Sci. 2015, 6, 2385–2392. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.; Valente, L.; Pinto, J.E.; Bertolucci, S.; Bezerra, G.; Alves, F.; Santos, P.; Benevides, P.; Siani, A.; Rosario, S.; et al. In Vitro Cultivated Uncaria tomentosa and Uncaria guianensis with Determination of the Pentacyclic Oxindole Alkaloid Contents and Profiles. J. Braz. Chem. Soc. 2008, 19, 1193–1200. [Google Scholar] [CrossRef]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant. 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Abbas, M.; Taha, H.; Aly, U.; El-Shabrawi, H.; Gaber, E.-S. In vitro propagation of ginger (Zingiber officinale Rosco). J. Gene. Eng. Biotechnol. 2011, 9, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.; Erickson, J.; Lloyd, B.; Slavin, J. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682–1688. [Google Scholar] [CrossRef]
- López, M.G.; Urias-Silvas, J. Agave fructans as prebiotics. In Recent Advances in Fructooligosaccharides Research; Norio, S., Noureddine, B., Shuichi, O., Eds.; Research Signpost: Trivandrum, Kerala, India 2007; pp. 297–310. [Google Scholar]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods. J. Funct. Food 2013, 1542–1553. [Google Scholar] [CrossRef]
- Melilli, M.G.; Branca, F.; Sillitti, C.; Scandurra, S.; Calderaro, P.; Di Stefano, V. Germplasm evaluation to obtain inulin with high degree of polymerization in Mediterranean environment. Nat. Prod. Res. 2020, 34, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ozsan, T.; Onus, A.N. Comparative study on in vitro micropropagation response of seven globe artichoke Cynara cardunculus var. scolymus (L.) Fiori cultivars: Open-pollinated cultivars vs F-1 hybrids. Not. Bot. Horti. Agrobot. Cluj Napoca 2020, 48, 1210–1220. [Google Scholar] [CrossRef]
- Caetano, B.; Sivieri, K.; Antunes de Moura, N. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Costa, C.; Conte, A.; Melilli, M.G.; Sillitti, C.; Bognanni, R.; Raccuia, S.A.; Del Nobile, M.A. The quality of functional whole-meal durum wheat spaghetti as affected by inulin polymerization degree. Carbohydr. Polym. 2017, 173, 84–90. [Google Scholar] [CrossRef]
- Global Market Insights. Available online: https://www.gminsights.com/industry-analysis/inulin-market#:~:text=Global%20inulin%20market%20size%20was,USD%202.5%20billion%20by%202023 (accessed on 3 August 2020).
- Genta, S.; Habib, N.; Pons, J.; Manrique, I.; Grau, A.; Sanchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. ESPEN 2009, 28, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.; Manrique, I.; Degen, L.; Beglinger, C. Effect of Yacon (Smallanthus sonchifolius) on Colonic Transit Time in Healthy Volunteers. Digestion 2008, 78, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriano, L.; Dionísio, A.; Abreu, F.; Carioca, A.A.F.; Zocolo, G.; Wurlitzer, N.; Pinto, C.; Oliveira, A.; Sampaio, H. Yacon syrup reduces postprandial glycemic response to breakfast: A randomized, crossover, double-blind clinical trial. Int. Food. Res. J. 2019, 126, 108682. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Silva, N.; Chaves, J.; Alfenas, R. Consumption of yacon flour improves body composition and intestinal function in overweight adults: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2018, 29, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumiyanto, J.; Dayan, F.; Cerdeira, A.; Wang, Y.-H.; Khan, I.; Moraes, R. Oligofructans content and yield of yacon (Smallanthus sonchifolius) cultivated in Mississippi. Sci. Hortic. 2012, 148, 83–88. [Google Scholar] [CrossRef]
- Robert, M.L.; Herrera-Herrera, J.L.; Castilho, C.; Ojeda, G.; Herrera-Amillo, M.A. An Efficient method for the micropropagation of Agave Species. Methods Mol. Biol. 2006, 318, 165–178. [Google Scholar] [PubMed]
- Prevati, A.; Benelli, C.; Re, F.D.; Carlo, A.; Vettori, C.; Lambardi, M. In vitro propagation and conservation of red chicory germplasm. In Proceedings of the The Role of Biotechnology, Turim, Italy, 5–7 March 2005; pp. 207–208. [Google Scholar]
- Dolinski, R.; Olek, A. Micropropagation of wild chicory (Cichorium intybus L. var. silvestre bisch.) from leaf explants. Acta Sci. Pol. Hortorum Cultus 2013, 12, 33–44. [Google Scholar]
- Abdalla, N.A. Micropropagation of Jerusalem Artichoke (Helianthus tuberosus L.) Plant. Ph.D. Thesis, Department of Horticulture Faculty of Agriculture, Ain Shams University, Cairo, Egypt, 2020. [Google Scholar]
- Viehmannova, I.; Bortlova, Z.; Vitamvas, J.; Cepkova, P.H.; Eliasova, K.; Svobodova, E.; Travnickova, M. Assessment of somaclonal variation in somatic embryo-derived plants of yacon [Smallanthus sonchifolius (Poepp. And Endl.) H. Robinson] using inter simple sequence repeat analysis and flow cytometry. Electron. J. Biotechnol. 2014, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Wildman, R.E.C. Nutraceuticals: A Brief review of historical and teleological aspects. In Hand Book of Nutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: London, UK, 2001. [Google Scholar]
- Royston, K.I.; Tollefsbol, T.O. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef]
- Melilli, M.G.; Pagliaro, A.; Scandurra, S.; Gentile, C.; Di Stefano, V. Omega-3 rich foods: Durum wheat spaghetti fortified with Portulaca oleracea. Food Biosci. 2020, 37. [Google Scholar] [CrossRef]


| Plant Species | Natural Substance | Therapy | Micropropagation Protocol |
|---|---|---|---|
| Artemisia annua L. | Artemisinin | Antimalarial | Etienne et al. [34] |
| Catharanthus roseus (L.) G. Don | Vincristine, Vinblastine | Anticancer | Kumar et al. [35] |
| Campotheca acuminata Decne | Camptothecin | Anticancer, antiviral | Nacheva et al. [36] |
| Leucojum aestivum L. | Galantamine | Anti-alzheimer | Zagorska et al. [37] |
| Narcissus sp. L. | Galantamine | Anti-alzheimer | Khonakdari et al. [38] |
| Hyoscyamus niger L. | Scopolamine | Parasympatholytic | Uranbey et al. [39] |
| Pilocarpus sp. Vahl | Pilocarpine | Anti-glaucoma | Saba et al. [40] |
| Podophyllum emodi Wall ex Royle | Podophyllotoxin | Anticancer, antiviral | Chakraborty et al. [41] |
| Podophyllum peltatum L. | Podophyllotoxin | Anticancer, antiviral | Moraes-Cerdeira et al. [5] |
| Rauwolfia serpentina (L.) Benth ex Kurz | Reserpine | Hypotensive, sedative | Bhatt et al. [42] |
| Taxus sp L. | Paclitaxel | Anticancer | Abbasin et al. [43] |
| Plant Species (Common Name) | Herbal Constituents | Therapy | Micropropation Protocol |
|---|---|---|---|
| Actaea racemosa L. (Black cohosh) | Triterpenes | Hot flashes menopause | Lata et al. [57] |
| Aesculus hippocastanum L. (Horse chestnut) | Coumarins (Aesculetin), Triterpenoid Saponin Glycoside | Varicose vein syndrome | Sediva et al. [58] |
| Allium sativum L. (Garlic) | Thiosulfinates (Allicin), Terpenes | Bronchitis, asthma, arteriosclerosis | Ayabe and Sumi [59] |
| Aloe vera (L.) Burm. f. (Aloe) | Polysaccharides | Laxative, healing burns and wounds | Roy and Sarka [60] |
| Calendula officinalis L. (Calendula) | Flavonoids, Terpenes, | Anti-inflammatory, healing wounds | Çöçü et al. [61] |
| Cynara scolymus L. (Artichoke) | Flavonoids, Caffeoylquinic Acids | Hepatic-biliary, dysfunction and digestive complaints | El Boullani et al. [62] |
| Echinacea purpurea (L.) Moench (Echinacea) | Alkamides, Cichoric Acid, Polysaccharides | Cold treatment | Jones et al. [63] |
| Ginkgo biloba L. (Ginkgo) | Flavonoids, Terpene lactones | Circulatory disorders | Camper et al. [64] |
| Harpagophytum procumbens DC. ex Meisn. (Devil’s claw) | Iridoid glycosides | Anti-inflammatory | Kaliamoorthy et al. [65] |
| Hypericum perforatum L. (St. John’s wort) | Naphthodianthrones (Hypericin, pseudohypericin) | Antidepressant | Gadzovska et al. [66] |
| Lippia sidoides Cham. (Pepper rosmarin) | Essential Oils | Anti-inflammatory, antifungal, antiseptic | Costa et al. [54] |
| Matricaria chamomilla L. (Camomile) | Flavonoids, Essential Oils | antispasmodic, anti-inflammatory | Taniguchi & Tanakano [67] |
| Maytenus ilicifolia Mart. (Espinheira santa) | Flavonoids, Triterpenes | Gastric disordes | Pereira et al. [68] |
| Passiflora incarnata L. (Passion flower) | Flavonoids, Coumarin, Umbelliferone, Indol Alkaloids | Anxiolytic | Ozarowski &Thiem [69] |
| Paullinia cupana Kunth (Guaraná) | Caffeine | CNS stimmulant, antioxidant | Barbosa & Mendes [70] |
| Peumus boldus Molina (Boldo) | Essential oils, Aporphine Alkaloid, Flavonoids | Hepatic, diuretic, laxative | Rios et al. [71] |
| Piper methysticum G. Forst (Kava-kava) | Kavalactones | CNS activity, antidepression, anxiolytic | Zhang et al. [72] |
| Psidium guajava L. (Guava) | Tannins, Flavonoids, Triterpenes | Noninfectious diarrhea | Rawls et al. [73] |
| Stryphnodendron adstringens (Mart.) Coville (Barbatimão) | Tannins | Wound healing | França et al. [10] |
| Uncaria tomentosa (Willd. ex Schults) DC. (Cat’s claw) | Flavonoids, Alkaloids, Saponins, Triterpenes | Anti-inflammatory | Pereira et al. [74] |
| Valeriana officinailis L. (Valeriana) | Terpenes, Valepotriates, Lignans | Anxiolytic, insomnia, sedative | Abdi et al. [75] |
| Zingiber officinale Roscoe (Ginger) | Essential oils, Shogaol, Zingerone, Gingerol | Anti-inflammatory, anti-emetic and chemo-protective | Abbas et al. [76] |
| Plant Species | Common Name | Culture Purposes | Microprogation Protocol |
|---|---|---|---|
| Agave sp L. | Agave, maguey | Production of high yielding plants | Robert et al. [91] |
| Chicorium intybus L. Cuanara cardunculus var. scolymus L. | Chicory Globe artichoke | Germplasm conservation, Improve root quality for medicinal value Propagation of open-pollinated cultivars | Previati et al. [92] Dolinski and Olek [93] Ozsan and Onus [82] |
| Helianthus tuberosus L. | Jerusalem artichoke | Large scale production of health plantlets | Abdalla [94] |
| Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson | Yacon | Production of healthy plantlets | Viehmannova et al. [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, R.M.; Cerdeira, A.L.; Lourenço, M.V. Using Micropropagation to Develop Medicinal Plants into Crops. Molecules 2021, 26, 1752. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061752
Moraes RM, Cerdeira AL, Lourenço MV. Using Micropropagation to Develop Medicinal Plants into Crops. Molecules. 2021; 26(6):1752. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061752
Chicago/Turabian StyleMoraes, Rita M., Antonio Luiz Cerdeira, and Miriam V. Lourenço. 2021. "Using Micropropagation to Develop Medicinal Plants into Crops" Molecules 26, no. 6: 1752. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061752