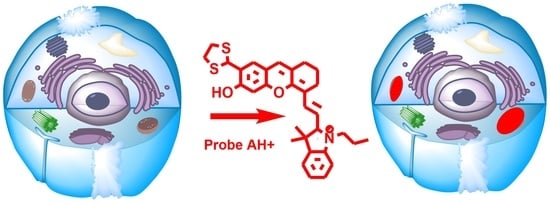
Ratiometric Near-Infrared Fluorescent Probes Based on Hemicyanine Dyes Bearing Dithioacetal and Formal Residues for pH Detection in Mitochondria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Probe Design and Synthesis
2.2. Optical Responses of Probes AH+ and BH+ to pH Changes
2.3. Theoretical Results
2.4. Assessing H+ Specificity of Probes AH+ and BH+
2.5. Photostability of the Probes
2.6. Cell Cytotoxicity
2.7. Analysis of Mitochondrial Localization for AH+ and BH+ Probes in Live Cells
2.8. Assessing AH+ and BH+ as Intercellular pH Sensors
3. Experimental Section
3.1. Computational Analysis of Probes A, AH+, B, and BH+
3.2. Reagents and Methods
3.3. Cell Culture and Cytotoxicity Assay
3.4. Cellular Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 5. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.N.; Ma, C.G.; Zhang, P.; Fu, Y.Q.; Shen, B.X. Recent progress in the development of fluorescent probes for detection of biothiols. Dyes Pigment. 2020, 177, 108321. [Google Scholar] [CrossRef]
- Wu, L.L.; Huang, C.S.; Emery, B.; Sedgwick, A.C.; Bull, S.D.; He, X.P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Forster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.L.; Du, J.J.; Peng, X.J. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Yue, Y.K.; Huo, F.J.; Lee, S.; Yin, C.X.; Yoon, J. A review: The trend of progress about pH probes in cell application in recent years. Analyst 2017, 142, 30–41. [Google Scholar] [CrossRef]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef]
- Liu, Y.C.; Teng, L.L.; Chen, L.L.; Ma, H.C.; Liu, H.W.; Zhang, X.B. Engineering of a near-infrared fluorescent probe for real-time simultaneous visualization of intracellular hypoxia and induced mitophagy. Chem. Sci. 2018, 9, 5347–5353. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.H.; Jung, Y.N.; Lin, L.J.; Liu, C.; Wu, C.; Cann, I.K.O.; Ha, T. Quantitative Fluorescent Labeling of Aldehyde-Tagged Proteins for Single-Molecule Imaging. Biophys. J. 2012, 102, 581A. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.N.; Xiong, F.; Peng, J.Z.; Fung, Y.M.E.; Huang, Y.R.; Li, X.Y. Introducing aldehyde functionality to proteins using ligand-directed affinity labeling. Chem. Commun. 2020, 56, 6134–6137. [Google Scholar] [CrossRef]
- Wu, P.; Shui, W.Q.; Carlson, B.L.; Hu, N.; Rabuka, D.; Lee, J.; Bertozzi, C.R. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc. Natl. Acad. Sci. USA 2009, 106, 3000–3005. [Google Scholar] [CrossRef] [Green Version]
- Purushottam, L.; Adusumalli, S.R.; Singh, U.; Unnikrishnan, V.B.; Rawale, D.G.; Gujrati, M.; Mishra, R.K.; Rai, V. Single-site glycine-specific labeling of proteins. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.A.; Wang, J.B.; Zhang, Y.B.; Whisman, N.; Bi, J.H.; Steenwinkel, T.E.; Wan, S.L.; Medford, J.; Tajiri, M.; Luck, R.L.; et al. Ratiometric fluorescent probes based on through-bond energy transfer of cyanine donors to near-infrared hemicyanine acceptors for mitochondrial pH detection and monitoring of mitophagy. J. Mater. Chem. B 2020, 8, 1603–1615. [Google Scholar] [CrossRef]
- Li, X.Y.; Hu, Y.M.; Li, X.H.; Ma, H.M. Mitochondria-Immobilized Near-Infrared Ratiometric Fluorescent pH Probe To Evaluate Cellular Mitophagy. Anal. Chem. 2019, 91, 11409–11416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.B.; Dong, Y.Q.; Zhou, J.; Zhou, Z.Y.; Wu, X.Z.; Wang, R.Z.; Miao, Z.C.; Liu, Y.Y.; Zhuo, S.P. Monitoring mitochondrial pH with a hemicyanine-based ratiometric fluorescent probe. Analyst 2019, 144, 3422–3427. [Google Scholar] [CrossRef]
- Ning, P.; Hou, L.L.; Feng, Y.; Xu, G.Y.; Bai, Y.Y.; Yu, H.Z.; Meng, X.M. Real-time visualization of autophagy by monitoring the fluctuation of lysosomal pH with a ratiometric two-photon fluorescent probe. Chem. Commun. 2019, 55, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.J.; Kim, K.; Park, K.S.; Kang, J.S.; Lim, C.S.; Kim, H.M.; Kang, C.; Lee, M.H. High-depth fluorescence imaging using a two-photon FRET system for mitochondrial pH in live cells and tissues. Chem. Commun. 2018, 54, 13531–13534. [Google Scholar] [CrossRef]
- Sarkar, A.R.; Heo, C.H.; Xu, L.; Lee, H.W.; Si, H.Y.; Byun, J.W.; Kim, H.M. A ratiometric two-photon probe for quantitative imaging of mitochondrial pH values. Chem. Sci. 2016, 7, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Zhu, C.C.; Cen, J.J.; Bai, Y.; He, W.J.; Guo, Z.J. Ratiometric detection of pH fluctuation in mitochondria with a new fluorescein/cyanine hybrid sensor. Chem. Sci. 2015, 6, 3187–3194. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Li, K.; Liu, Y.H.; Yu, K.K.; Xie, Y.M.; Zhou, X.D.; Yu, X.Q. Mitochondria-targeted ratiometric fluorescent probe for real time monitoring of pH in living cells. Biomaterials 2015, 53, 669–678. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Xia, S.; Mikesell, L.; Whisman, N.; Fang, M.X.; Steenwinkel, T.E.; Chen, K.; Luck, R.L.; Werner, T.; Liu, H.Y. Near-Infrared Hybrid Rhodol Dyes with Spiropyran Switches for Sensitive Ratiometric Sensing of pH Changes in Mitochondria and Drosophila melanogaster First-Instar Larvae. ACS Appl. Bio Mater. 2019, 2, 4986–4997. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Guo, L.; Devarajan, D.; Parks, J.M.; Painter, S.L.; Brooks, S.C.; Smith, J.C. The AQUA-MER databases and aqueous speciation server: A web resource for multiscale modeling of mercury speciation. J. Comput. Chem. 2020, 41, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Johnston, R.C.; Parks, J.M.; Smith, J.C. Quantum Chemical Calculation of pKa’s of Environmentally Relevant Functional Groups: Carboxylic Acids, Amines, and Thiols in Aqueous Solution. J. Phys. Chem. A 2018, 122, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Thapa, B.; Schlegel, H.B. Improved pKa Prediction of Substituted Alcohols, Phenols, and Hydroperoxides in Aqueous Medium Using Density Functional Theory and a Cluster-Continuum Solvation Model. J. Phys. Chem. A 2017, 121, 4698–4706. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rhee, W.J.; Bao, G. Slow non-specific accumulation of 2’-deoxy and 2’-O-methyl oligonucleotide probes at mitochondria in live cells. Nucleic Acids Res. 2010, 38, e109. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.W.; Liu, H.X.; He, H.; Yadava, N.; Chambers, J.J.; Thayumanavan, S. Anionic Polymers Promote Mitochondrial Targeting of Delocalized Lipophilic Cations. Bioconjug. Chem. 2020, 31, 1344–1353. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef]
- Poburko, D.; Santo-Domingo, J.; Demaurex, N. Dynamic Regulation of the Mitochondrial Proton Gradient during Cytosolic Calcium Elevations. J. Biol. Chem. 2011, 286, 11672–11684. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, J.; Wang, L.L.; Hu, X.X.; Liu, X.J.; Liu, M.R.; Cao, Z.H.; Shangguan, D.H.; Tan, W.H. A Cyanine Dye to Probe Mitophagy: Simultaneous Detection of Mitochondria and Autolysosomes in Live Cells. J. Am. Chem. Soc. 2016, 138, 12368–12374. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Bi, J.H.; Xia, S.; Mazi, W.; Wan, S.L.; Mikesell, L.; Luck, R.L.; Liu, H.Y. A Near-Infrared Fluorescent Probe Based on a FRET Rhodamine Donor Linked to a Cyanine Acceptor for Sensitive Detection of Intracellular pH Alternations. Molecules 2018, 23, 2679. [Google Scholar] [CrossRef] [Green Version]
- Mazi, W.; Yan, Y.N.; Zhang, Y.B.; Xia, S.; Wan, S.L.; Tajiri, M.; Luck, R.L.; Liu, H.Y. A near-infrared fluorescent probe based on a hemicyanine dye with an oxazolidine switch for mitochondrial pH detection. J. Mater. Chem. B 2021, 9, 857–863. [Google Scholar] [CrossRef]
- Xia, S.; Wang, J.B.; Bi, J.H.; Wang, X.; Fang, M.X.; Phillips, T.; May, A.; Conner, N.; Tanasova, M.; Luo, F.T.; et al. Fluorescent probes based on pi-conjugation modulation between hemicyanine and coumarin moieties for ratiometric detection of pH changes in live cells with visible and near-infrared channels. Sens. Actuator B-Chem. 2018, 265, 699–708. [Google Scholar] [CrossRef]
- Wang, J.B.; Xia, S.; Bi, J.H.; Zhang, Y.B.; Fang, M.X.; Luck, R.L.; Zeng, Y.B.; Chen, T.H.; Lee, H.M.; Liu, H.Y. Near-infrared fluorescent probes based on TBET and FRET rhodamine acceptors with different pK(a) values for sensitive ratiometric visualization of pH changes in live cells. J. Mater. Chem. B 2019, 7, 198–209. [Google Scholar] [CrossRef]
- Xia, S.; Fang, M.X.; Wang, J.B.; Bi, J.H.; Mazi, W.; Zhang, Y.B.; Luck, R.L.; Liu, H.Y. Near-infrared fluorescent probes with BODIPY donors and rhodamine and merocyanine acceptors for ratiometric determination of lysosomal pH variance. Sens. Actuator B-Chem. 2019, 294, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Dong, H.; Yu, M.M.; Liu, C.X.; Li, Z.X.; Wei, L.H.; Sun, L.D.; Zhang, H.Y. NIR Ratiometric Luminescence Detection of pH Fluctuation in Living Cells with Hemicyanine Derivative-Assembled Upconversion Nanophosphors. Anal. Chem. 2017, 89, 8863–8869. [Google Scholar] [CrossRef]
- Wang, J.B.; Xia, S.; Bi, J.H.; Fang, M.X.; Mazi, W.F.; Zhang, Y.B.; Conner, N.; Luo, F.T.; Lu, H.P.; Liu, H.Y. Ratiometric Near-Infrared Fluorescent Probes Based On Through Bond Energy Transfer and pi-Conjugation Modulation between Tetraphenylethene and Hemicyanine Moieties for Sensitive Detection of pH Changes in Live Cells. Bioconjug. Chem. 2018, 29, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]



















| Probe | pH | Absmax (nm) | Emmax (nm) | ε (105 M−1 cm−1) | Φf | Δλfl (nm) | pKa |
|---|---|---|---|---|---|---|---|
| AH+ | 4.0 | 658 | 680 | 0.21 | 0.06 | 22 | 6.85 |
| 9.2 | 701 | 718 | 0.17 | 0.27 | |||
| BH+ | 4.0 | 586 | 667 | 0.42 | 1.9 | 81 | 6.49 |
| 9.2 | 684 | 715 | 0.36 | 12.3 |
| Probe | Experimental | IEF-PCM a | SMD b | SMD c (H2O) d | Bondi | Bondi(H2O) | SAS | SAS(H2O) |
|---|---|---|---|---|---|---|---|---|
| AH+ | 6.85 | 6.37 | 8.52 | 8.29 (8.66) | 8.54 | 7.33(7.57) | 9.64 | 8.60 (9.76) |
| BH+ | 6.49 | 0.26 | 6.48 | 6.27 | 5.81 | 6.14 | 7.60 | 6.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, Y.; Xia, S.; Wan, S.; Vohs, T.; Tanasova, M.; Luck, R.L.; Liu, H. Ratiometric Near-Infrared Fluorescent Probes Based on Hemicyanine Dyes Bearing Dithioacetal and Formal Residues for pH Detection in Mitochondria. Molecules 2021, 26, 2088. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072088
Yan Y, Zhang Y, Xia S, Wan S, Vohs T, Tanasova M, Luck RL, Liu H. Ratiometric Near-Infrared Fluorescent Probes Based on Hemicyanine Dyes Bearing Dithioacetal and Formal Residues for pH Detection in Mitochondria. Molecules. 2021; 26(7):2088. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072088
Chicago/Turabian StyleYan, Yunnan, Yibin Zhang, Shuai Xia, Shulin Wan, Tara Vohs, Marina Tanasova, Rudy L. Luck, and Haiying Liu. 2021. "Ratiometric Near-Infrared Fluorescent Probes Based on Hemicyanine Dyes Bearing Dithioacetal and Formal Residues for pH Detection in Mitochondria" Molecules 26, no. 7: 2088. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072088