Catalytic Conversion of Xylose to Furfural by p-Toluenesulfonic Acid (pTSA) and Chlorides: Process Optimization and Kinetic Modeling
Abstract
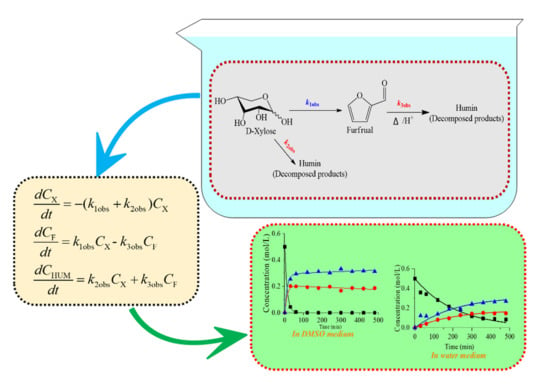
:1. Introduction
2. Results and Discussion
2.1. Aqueous Phase Dehydration of Xylose into Furfural
2.2. Effect of Lewis Acid Addition
2.3. Conversion of Xylose to Furfural in Organic Medium
2.4. Comparison of Different Organic Acids
3. Materials and Methods
3.1. Materials
3.2. Conversion of Xylose to Furfural
4. Analytics
4.1. Product Quantification
4.2. Development of Kinetic Models
4.3. Estimation of Kinetic Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bender, T.A.; Dabrowski, J.A.; Gagné, M.R. Homogeneous catalysis for the production of low-volume, high-value chemicals from biomass. Nat. Rev. Chem. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Tan, M.; Ma, L.; Rehman, M.S.U.; Ahmed, M.A.; Sajid, M.; Xu, X.; Sun, Y.; Cui, P.; Xu, J. Screening of acidic and alkaline pretreatments for walnut shell and corn stover biorefining using two way heterogeneity evaluation. Renew. Energy 2019, 132, 950–958. [Google Scholar] [CrossRef]
- Dulie, N.W.; Woldeyes, B.; Demsash, H.D.; Jabasingh, A.S. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Sachdev, N.; Singh, A.K.; Shrivastava, A.; Katre, Y. Kinetic and mechanistic investigations of chlorocomplex of Ru(III) and Ir(III) catalyzed oxidation of D-fructose by N-bromophthalimide in acidic medium. J. Saudi Chem. Soc. 2016, 20, S357–S375. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Guo, H.; Xie, Q.; Wang, J.; Hou, B.; Jia, L.; Cui, J.; Li, D. Conversion of fructose into furfural or 5-hydroxymethylfurfural over HY zeolites selectively in γ-butyrolactone. Appl. Catal. A Gen. 2019, 572, 51–60. [Google Scholar] [CrossRef]
- Padilla-Rascón, C.; Romero-García, J.M.; Ruiz, E.; Castro, E. Optimization with response surface methodology of microwave-assisted conversion of xylose to furfural. Molecules 2020, 25, 3574. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Danon, B.; Hongsiri, W.; van der Aa, L.; de Jong, W. Kinetic study on homogeneously catalyzed xylose dehydration to furfural in the presence of arabinose and glucose. Biomass Bioenergy 2014, 66, 364–370. [Google Scholar] [CrossRef]
- Sener, C.; Motagamwala, A.H.; Alonso, D.M.; Dumesic, J.A. Enhanced Furfural Yields from Xylose Dehydration in the γ-Valerolactone/Water Solvent System at Elevated Temperatures. ChemSusChem 2018, 11, 2321–2331. [Google Scholar] [CrossRef]
- Yemiş, O.G.M. Catalytic Performances of Various Solid Catalysts and Metal Halides for Microwave-Assisted Hydrothermal Conversion of Xylose, Xylan, and Straw to Furfural. Waste Biomass Valorization 2019, 10, 1343–1353. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.B.; Victor, A.; Pulidindi, I.N.; Gedanken, A. Selective production of furfural from the dehydration of xylose using Zn doped CuO catalyst. Ultrason. Sonochem. 2019, 56, 55–62. [Google Scholar] [CrossRef]
- Lamminpää, K.; Ahola, J.; Tanskanen, J.; Lamminpä, K.; Ahola, J.; Tanskanen, J. Kinetics of Xylose Dehydration into Furfural in Formic Acid. Ind. Eng. Chem. Res. 2012, 51, 6297–6303. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Fan, J.; Wu, N.; Gao, L.; Li, W.; Xiao, G. Catalytic Conversion of Xylose and Xylan into Furfural over Cr3+/P-SBA-15 Catalyst Derived from Spent Adsorbent. Ind. Eng. Chem. Res. 2019, 58, 13013–13020. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.E.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives-a review of recent advances in the production of furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S.S.S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Modelska, M.; Berlowska, J.; Kregiel, D.; Cieciura, W.; Antolak, H.; Tomaszewska, J.; Binczarski, M.; Szubiakiewicz, E.; Witonska, I. Concept for Recycling Waste Biomass from the Sugar Industry for Chemical and Biotechnological Purposes. Molecules 2017, 22, 1544. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, Y.; Lin, H.; Chen, J.; Zhu, L.; Luo, Z. Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone. Green Chem. 2017, 19, 3869–3879. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Gómez Millán, G.; Hellsten, S.; King, A.W.T.; Pokki, J.-P.; Llorca, J.; Sixta, H. A comparative study of water-immiscible organic solvents in the production of furfural from xylose and birch hydrolysate. J. Ind. Eng. Chem. 2019, 72, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Bachosz, K.; Synoradzki, K.; Staszak, M.; Pinelo, M.; Meyer, A.S.; Zdarta, J.; Jesionowski, T. Bioconversion of xylose to xylonic acid via co-immobilized dehydrogenases for conjunct cofactor regeneration. Bioorg. Chem. 2019, 93, 102747. [Google Scholar] [CrossRef]
- Zdarta, J.; Bachosz, K.; Degórska, O.; Zdarta, A.; Kaczorek, E.; Pinelo, M.; Meyer, A.S.; Jesionowski, T. Co-immobilization of glucose dehydrogenase and xylose dehydrogenase as a new approach for simultaneous production of gluconic and xylonic acid. Materials 2019, 12, 3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Hewetson, B.B.; Mosier, N.S. Kinetics of Maleic Acid and Aluminum Chloride Catalyzed Dehydration and Degradation of Glucose. Energy Fuels 2015, 29, 2387–2393. [Google Scholar] [CrossRef]
- Ji, H.; Chen, L.; Zhu, J.Y.; Gleisner, R.; Zhang, X. Reaction Kinetics Based Optimization of Furfural Production from Corncob Using a Fully Recyclable Solid Acid. Ind. Eng. Chem. Res. 2016, 55, 11253–11259. [Google Scholar] [CrossRef]
- O’Neil, R.; Ahmad, M.N.; Vanoye, L.; Aiouache, F. Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 zeolite. Ind. Eng. Chem. Res. 2009, 48, 4300–4306. [Google Scholar] [CrossRef]
- Marcotullio, G.; De Jong, W. Chloride ions enhance furfural formation from d-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, R.-C. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, R.; Cho, J.; Conner, W.C., Jr.; Huber, G.W.; Conner, W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Morinelly, J.E.; Jensen, J.R.; Browne, M.; Co, T.B.; Shonnard, D.R. Kinetic characterization of xylose monomer and oligomer concentrations during dilute acid pretreatment of lignocellulosic biomass from forests and switchgrass. Ind. Eng. Chem. Res. 2009, 48, 9877–9884. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Xu, J.; Li, P. Kinetics of xylose dehydration into furfural in acetic acid. Chin. J. Chem. Eng. 2015, 23, 659–666. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, Y.; Liu, D.; Zhao, X. Conversion of Glucose to 5-Hydroxymethylfurfural by Co-catalysis of p-Toluenesulfonic Acid (pTSA) and Chlorides: A Comparison Based on Kinetic Modeling. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, Y.; Liu, D.; Zhao, X. Organic acid catalyzed production of platform chemical 5-hydroxymethylfurfural from fructose: Process comparison and evaluation based on kinetic modeling. Arab. J. Chem. 2020, 13, 7430–7444. [Google Scholar] [CrossRef]
- Köchermann, J.; Schreiber, J.; Klemm, M. Conversion of d-Xylose and Hemicellulose in Water/Ethanol Mixtures. ACS Sustain. Chem. Eng. 2019, 7, 12323–12330. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of xylose to furfural using Lewis and Brønsted acid catalysts in aqueous media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. The Role of Metal Halides in Enhancing the Dehydration of Xylose to Furfural. ChemCatChem 2015, 7, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ren, J.; Li, H.; Deng, A.; Sun, R. Direct transformation of xylan-type hemicelluloses to furfural via SnCl4 catalysts in aqueous and biphasic systems. Bioresour. Technol. 2015, 183, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Dallas Swift, T.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef] [Green Version]
- Weiqi, W.; Shubin, W. Experimental and kinetic study of glucose conversion to levulinic acid catalyzed by synergy of Lewis and Brønsted acids. Chem. Eng. J. 2017, 307, 389–398. [Google Scholar] [CrossRef]
- Danon, B.; Marcotullio, G.; De Jong, W. Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem. 2014, 16, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Guo, F.; Li, Y.; Zheng, Z.; Xing, Z.; Zhu, Z.; Liu, T.; Zhang, X.; Jin, Y. Dehydration of D-xylose into furfural over bimetallic salts of heteropolyacid in DMSO/H2O mixture. Appl. Catal. A Gen. 2018, 558, 18–25. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Jing, Q.; Lu, X.; Yuan, L. Kinetics of non-catalyzed decomposition of xylose in high temperature liquid water. Huaxue Fanying Gongcheng Yu Gongyi/Chem. React. Eng. Technol. 2006, 22, 472–475. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, X.; Du, W.; Liu, D.; Xiaoying, S.; Xuebing, Z.; Wei, D.; Dehua, L. Kinetics of Formic Acid-autocatalyzed Preparation of Performic Acid in Aqueous Phase. Chin. J. Chem. Eng. 2011, 19, 964971. [Google Scholar] [CrossRef]
- Dunlop, A.P. Furfural Formation and Behavior. Ind. Eng. Chem. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.W.; Abu-Omar, M.M. Synthesis of Furfural from Xylose, Xylan, and Biomass Using AlCl3·6H2O in Biphasic Media via Xylose Isomerization to Xylulose. ChemSusChem 2012, 5, 405–410. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent advances in catalytic hydrogenation of furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. 2014, 53, 11872–11875. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural. Energies 2018, 11, 645. [Google Scholar] [CrossRef] [Green Version]






| Solvent | Rate Constant (min−1) | Goodness of Fit (R2) | SF | Max Yield | ||||
|---|---|---|---|---|---|---|---|---|
| k1obs | k2obs | k3obs | Xylose | Furfural | Humin | k1obs/k2obs | Ymax | |
| DMF | 0.029 | 0.053 | 2.04 × 10−3 | 0.997 | 0.961 | 0.989 | 0.547 | 29.74 ± 1.1 |
| DMSO | 0.033 | 0.047 | 3.20 × 10−4 | 0.999 | 0.973 | 0.990 | 0.701 | 40.35 ± 1.6 |
| Water | 1.70 × 10−3 | 2.97 × 10−3 | 5.21 × 10−11 | 0.974 | 0.986 | 0.922 | 0.572 | 30.1 ± 0.9 |
| Temp (°C) | Rate Constant (min−1) | Goodness of Fit (R2) | Max Yield | tYmax | ||||
|---|---|---|---|---|---|---|---|---|
| k1obs | k2obs | k3obs | Xylose | Furfural | Humin | Ymax | min | |
| 110 | 0.0208 | 0.0220 | 1.70 × 10−4 | 0.9990 | 0.979 | 0.988 | 33.5 ± 1.1 | 240 |
| 120 | 0.033 | 0.047 | 3.70 × 10−4 | 0.9999 | 0.973 | 0.990 | 40.4 ± 1.6 | 30 |
| 130 | 0.0854 | 0.0549 | 9.45 × 10−4 | 0.9999 | 0.997 | 0.999 | 34.1 ± 1.1 | 30 |
| 140 | 0.1286 | 0.1368 | 2.57 × 10−3 | 0.9999 | 0.919 | 0.973 | 33.8 ± 1.3 | 15 |
| 150 | 0.1740 | 0.1866 | 4.03 × 10−3 | 0.9999 | 0.995 | 0.999 | 34.4 ± 1.7 | 15 |
| 160 | 0.2792 | 0.2948 | 2.72 × 10−3 | 0.9999 | 0.931 | 0.983 | 34.8 ± 1.2 | 15 |
| CrCl3·6H2O (M) | Rate Constant (min−1) | Goodness of Fit (R2) | Max Yield | tYmax (min) | SF | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1obs | k2obs | k3obs | Xylose | Furfural | Humin | Ymax | |||
| 0.1 | 0.0508 | 0.0542 | 5.49 × 10−4 | 0.9990 | 0.9543 | 0.9538 | 52.6 ± 1.3 | 60 | 0.94 |
| 0.2 | 0.0509 | 0.0605 | 6.76 × 10−4 | 0.9999 | 0.9737 | 0.9797 | 48.4 ± 1.4 | 60 | 0.84 |
| 0.3 | 0.0360 | 0.0508 | 3.01 × 10−4 | 0.9999 | 0.9778 | 0.9905 | 45.1 ± 1.3 | 90 | 0.71 |
| 0.4 | 0.0349 | 0.0519 | 2.55 × 10−4 | 0.9999 | 0.9814 | 0.9929 | 43.1 ± 2.2 | 90 | 0.67 |
| 0.5 | 0.0330 | 0.047 | 3.70 × 10−4 | 0.9999 | 0.973 | 0.990 | 40.4 ± 1.6 | 30 | 0.70 |
| Organic Acid | Rate Constant (min−1) | Goodness of Fit (R2) | Max Yield | tYmax (h) | SF | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1obs | k2obs | k3obs | Xylose | Furfural | Humin | Ymax | |||
| pTSA | 0.0508 | 0.0542 | 5.49 × 10−4 | 0.9990 | 0.9543 | 0.9538 | 52.60 ± 1.3 | 1 | 0.94 |
| Oxalic acid | 0.0129 | 0.0183 | 1.30 × 10−12 | 0.967 | 0.983 | 0.8826 | 42.64 ± 1.2 | 5 | 0.71 |
| Succinic acid | 0.0056 | 0.0084 | 9.30 × 10−14 | 0.986 | 0.9718 | 0.8816 | 41.95 ± 1.5 | 5 | 0.66 |
| Maleic acid | 0.0062 | 0.0120 | 2.20 × 10−14 | 0.973 | 0.9 | 0.7568 | 37.1 ± 1.2 | 5 | 0.52 |
| Malonic acid | 0.0042 | 0.0081 | 2.20 × 10−14 | 0.885 | 0.9486 | 0.4205 | 32.41 ± 2.2 | 5 | 0.51 |
| kobs (min−1) | A | Ea (kJ/mol) | α | R2 |
|---|---|---|---|---|
| k1obs | 8.91 × 108 | 81.8 | 1.35517 | 0.855 |
| k2obs | 2.60 × 107 | 66.5 | 0.20398 | 0.962 |
| k3obs | 7.13 × 108 | 93.02 | 0.26768 | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, M.; Rizwan Dilshad, M.; Saif Ur Rehman, M.; Liu, D.; Zhao, X. Catalytic Conversion of Xylose to Furfural by p-Toluenesulfonic Acid (pTSA) and Chlorides: Process Optimization and Kinetic Modeling. Molecules 2021, 26, 2208. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082208
Sajid M, Rizwan Dilshad M, Saif Ur Rehman M, Liu D, Zhao X. Catalytic Conversion of Xylose to Furfural by p-Toluenesulfonic Acid (pTSA) and Chlorides: Process Optimization and Kinetic Modeling. Molecules. 2021; 26(8):2208. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082208
Chicago/Turabian StyleSajid, Muhammad, Muhammad Rizwan Dilshad, Muhammad Saif Ur Rehman, Dehua Liu, and Xuebing Zhao. 2021. "Catalytic Conversion of Xylose to Furfural by p-Toluenesulfonic Acid (pTSA) and Chlorides: Process Optimization and Kinetic Modeling" Molecules 26, no. 8: 2208. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082208