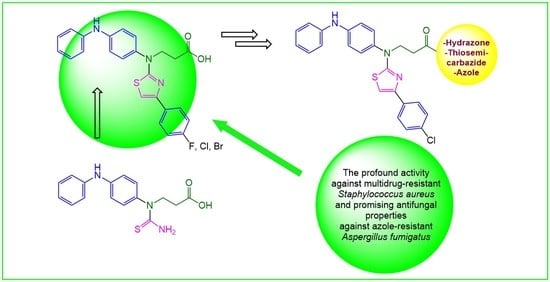
Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Characterization of Absorption, Distribution, Metabolism, and Excretion (ADME) Properties
2.3. Antimicrobial Activity of Compounds 1–9
2.4. The Cytotoxicity Evaluation of Compounds 1–9
2.5. The Compounds 2a–c Demonstrates Bactericidal Activity on Staphylococcus Aureus with Genetically Defined Resistance Mechanisms
2.6. The Activity of the Compounds 2a–c on the Staphylococcus Aureus Biofilm Integrity
3. Materials and Methods
3.1. Synthesis
- 3-(1-(4-(phenylamino)phenyl)thioureido)propanoic acid (1). A solution of thioxo tetrahydro pyrimidinone [35] (0.1 mol, 29.7 g) in aqueous 10% sodium hydroxide (200 mL) was boiled, then left to cool down to room temperature, filtered off, and the obtained filtrate was acidified with acetic acid to pH 6. The formed precipitate was filtered off, washed with water, and dried to give the title compound 1 (white solid, yield 20.2 g (64%), m.p. 195 °C (decomp.).
- General procedure for the preparation of thiazoles2a–c. A suspension of compound 1 (2.2 mmol) in aqueous 5% sodium carbonate solution (10 mL) was dissolved in methanol (6 mL), and the appropriate acetophenone (2.7 mmol) was added. The mixture was refluxed for 2 h (a, b) or 4 h (c), the cooled down and acidified with diluted (30%) acetic acid to pH 6. The formed precipitate was filtered off, washed with water and recrystallized from propan-2-ol to give the title compounds 2a (white solid, yield 0.65 g, 68%, m. p. 124–125 °C), 2b (white solid, yield 0.87 g, 88%, m. p. 130–132 °C), and 2c (white solid, yield 0.87 g, 80%, m.p. 120–122 °C).
- 3-((4-(4-Fluorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanoic acid(2a). 1H-NMR (400 MHz, DMSO-d6): δ = 2.62 (t, J = 7.4 Hz, 2H, CH2CO), 4.11 (t, J = 7.4 Hz, 2H, NCH2), 6.88 (t, J = 7.3, 1H, HAr), 7.08–7.17 (m, 5H, SCH, HAr), 7.22–7.30 (m, 4H, HAr), 7.44 (d, J = 8.5 Hz, 2H, HAr), 7.88 (d, J = 8.5 Hz, 2H, HAr), 8.39 (s, 1H, NH) ppm.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanoic acid(2b).1H-NMR (400 MHz, DMSO-d6): δ = 2.62 (t, J = 7.4 Hz, 2H, CH2CO), 4.11 (t, J = 7.4 Hz, 2H, NCH2), 6.88 (t, J = 7.3, 1H, HAr), 7.05–7.16 (m, 5H, SCH, HAr), 7.16–7.39 (m, 4H, HAr), 7.44 (d, J = 8.5 Hz, 2H, HAr), 7.88 (d, J = 8.5 Hz, 2H, HAr), 8.39 (s, 1H, NH) ppm.
- 3-((4-(4-Bromophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanoic acid(2c). 1H-NMR (400 MHz, DMSO-d6): δ = 2.44, 2.57 (2t, J = 7.8, 7.6 Hz, 2H, CH2CO), 4.09 (t, J = 7.6 Hz, 2H, NCH2), 6.75–7.34 (m, 10H, SCH, HAr), 7.58 (d, J = 8.2 Hz, 4H, HAr), 7.81 (d, J = 8.2 Hz, 4H, HAr), 8.33, 8.36 (2s, 1H, NH) ppm.
- Methyl 3-((4-(4-chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanoate(3). To a boiling solution of compound 2b (11.1 mmol, 5 g) in methanol (100 mL), a catalytic amount of sulfuric acid (0.6 mL) was added, and the reaction mixture was refluxed for 5 h. Then the volatile fractions were evaporated under reduced pressure, the residue was poured with aqueous 5% sodium carbonate solution (150 mL) and boiled. After cooling, the formed precipitate was filtered off, washed with water and recrystallized from the mixture of methanol and water (1:1) to give the title compound 4 (white solid, yield 4.12 g, 80%, m.p. 73–74 °C).
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanehydrazide(4). To a solution of ester 3 (20 mmol, 7.12 g) in propan-2-ol (150 mL), hydrazine monohydrate (60 mmol, 3 g) was added dropwise and the mixture was heated at reflux for 18 h. After completion of the reaction the mixture was cooled down and diluted with water (100 mL). The obtained precipitate was filtered off, washed with water, and recrystallized from propan-2-ol to give the title compound 4 (yellow solid, yield 7.52 g, 73%, m.p. 104–105 °C).
- General procedure for the preparation of hydrazones5a–i. To a boiling solution of hydrazide 4 (5 mmol, 2.32 g) in propan-2-ol (35 mL) the corresponding aromatic aldehyde (6 mmol) and a catalytic amount of acetic acid (0.4 mL) were added, and the mixture was heated at reflux for 2 h after, cooled down, the formed solid was filtered off, washed with propan-2-ol, diethyl ether, and recrystallized from propan-2-ol to give the appropriate title compound 5.
- N-benzylidene-3-((4-(4-chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanehydrazide(5a). White solid, yield 2.24 g, 81%, m.p. 162–164 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N′-(4-fluorobenzylidene)propanehydrazide (5b). White solid, yield 2.39 g, 84%, m.p. 178–180 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N′-(4-chlorobenzylidene)propanehydrazide (5c). White solid, yield 2.58 g, 88%, m.p. 201–203 °C.
- N′-(4-bromobenzylidene)-3-((4-(4-chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanehydrazide (5d) White solid, yield 2.78 g, 88%, m.p. 182–184 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)-N′-(4-nitrobenzylidene)-(4-(phenylamino)phenyl)amino)propanehydrazide (5e). White solid, yield 2.36 g, 79%, m.p. 205–207 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)-N′-(4-(dimethylamino)benzylidene)-(4-(phenylamino)phenyl)amino)propanehydrazide (5f). White solid, yield 2.38 g, 80%, m.p. 191–193 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)-N′-(4-methylbenzylidene)-(4-(phenylamino)phenyl)amino)propanehydrazide (5g). White solid, yield 2.69 g, 95%, m.p. 176–178 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N’-(thiophen-2-ylmethylene)propanehydrazide (5h). White solid, yield 1.90 g, 68%, m.p. 178–180 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N’-((5-nitrothiophen-2-yl)methylene)propanehydrazide (5i). White solid, yield 2.20 g, 73%, m.p. 186–188 °C.
- General procedure for the preparation of hydrazones5j, k. A mixture of hydrazide 4 (5 mmol, 2.32 g) and the corresponding ketone (acetone or 2-butanone) (35 mL) was heated at reflux for 5 h. After completion of the reaction, the mixture was cooled down, diluted with water (35 mL), and left in refrigerator for 24 h. Then the formed precipitate was filtered off, washed with acetone, diethyl ether and recrystallized from acetone to give the title compound 5j. Product 5k was separated from the reaction mixture by evaporating the volatile fractions under reduced pressure, diluting the residue with diethyl ether, filtering the obtained solid, and recrystallizing it from 2-butanone to give the title compound 5k.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N’-(propan-2-ylidene)propanehydrazide (5j). White solid, yield 2.07 g, 82%, m.p. 76–78 °C.
- N’-(butan-2-ylidene)-3-((4-(4-chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanehydrazide (5k). White solid, yield 1.76 g, 68%, m.p. 79–81 °C.
- 3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-1-(3,5-dimethyl-1H-pyrazol-1-yl)propan-1-one (6). To a solution of hydrazide 4 (5 mmol, 2.32 g) in propan-2-ol (35 mL), 2,4-pentanedione (10 mmol, 1 g) and hydrochloric acid (2 drops) were added dropwise and the mixture was refluxed for 9 h, then cooled down, and the formed precipitate was filtered off washed with propan-2-ol and recrystallized from propan-2-ol to give the title compound 7 (white solid, 1.95 g, 74%, m.p. 105–106 °C).
- 2-(3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)-N-(2,5-dimethyl-1H-pyrrol-1-yl)propanamide (7). To a solution of hydrazide 4 (5 mmol, 2.32 g) in propan-2-ol (45 mL) 2,5-hexanedione (9 mmol, 1.03 g) and acetic acid (dropwise, 0.75 mL) were added and the mixture was refluxed for 3 h, then cooled down and diluted with water (30 mL). The formed crystalline solid was filtered off, washed with water and recrystallized from propan-2-ol to give the title compound 7 (white solid, 2.17 g, 80%, m.p. 146–147 °C).
- 2-(3-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)propanoyl)-N-phenylhydrazine-1-carbothioamide (8). To a solution of hydrazide 4 (5 mmol, 2.32 g) solution in methanol (100 mL), a solution of phenyl isothiocyanate (5 mmol, 0.6 mL) in methanol (5 mL) was added dropwise and the mixture was refluxed for 2 h. After completion of the reaction, the mixture was cooled down and diluted with water (80 mL). The formed solid was filtered off, washed with water, and recrystallized from propan-2-ol to give the title compound 8 (white solid, 2.79 g, 93%, m.p. 116–118 °C).
- 5-(2-((4-(4-Chlorophenyl)thiazol-2-yl)(4-(phenylamino)phenyl)amino)ethyl)-4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9). A mixture of compound 8 (5 mmol, 3 g) and aqueous 4 % NaOH solution (150 mL) was heated at reflux for 3 h, then cooled down and acidified with acetic acid to pH 6. The formed precipitate was filtered off, washed with water and recrystallized from propan-2-ol to give the title compound 9 (white solid, 2.41 g, 83%, m.p. 119–121 °C).
3.2. In Silico ADME Prediction
3.3. Microbial Strains and Culture Conditions
3.4. Minimal Inhibitory Concentration (MIC) Determination
3.5. Evaluation of Cytotoxicity in Vero Cells
3.6. In Vitro Time-Kill Study
3.7. Biofilm Formation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kakkar, A.K.; Shafiq, N.; Singh, G.; Ray, P.; Gautam, V.; Agarwal, R.; Muralidharan, J.; Arora, P. Antimicrobial Stewardship Programs in Resource Constrained Environments: Understanding and Addressing the Need of the Systems. Front. Public Heal. 2020, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Al Maani, A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries: A position statement for the international society for infectious diseases. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Pharmacological Significance of Synthetic Heterocycles Scaffold: A Review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [Green Version]
- Radin, N.S. Drug design: Hiding in full view. Drug Dev. Res. 2008, 69, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Hano, Y.; Nomura, T.; Chen, Y. Novel quinazoline–quinoline alkaloids with cytotoxic and DNA topoisomerase II inhibitory activities. Bioorganic Med. Chem. Lett. 2004, 14, 1193–1196. [Google Scholar] [CrossRef]
- Santana, A.C.; Filho, R.C.S.; Menezes, J.C.; Allonso, D.; Campos, V.R. Nitrogen-Based Heterocyclic Compounds: A Promising Class of Antiviral Agents against Chikungunya Virus. Life 2020, 11, 16. [Google Scholar] [CrossRef]
- Sioriki, E.; Lordan, R.; Nahra, F.; Van Hecke, K.; Zabetakis, I.; Nolan, S.P. In vitro Anti-atherogenic Properties of N-Heterocyclic Carbene Aurate(I) Compounds. ChemMedChem 2018, 13, 2484–2487. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Dahiya, L.; Kumar, M. Fructose-1,6-bisphosphatase inhibitors: A new valid approach for management of type 2 diabetes mellitus. Eur. J. Med. Chem. 2017, 141, 473–505. [Google Scholar] [CrossRef]
- Goshain, O.; Ahmed, B. Antihypertensive activity, toxicity and molecular docking study of newly synthesized xanthon derivatives (xanthonoxypropanolamine). PLoS ONE. 2019, 14, e0220920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, K.; Surana, S.; Jain, P.; Patel, H.M. Mycobacterium Tuberculosis (MTB) GyrB inhibitors: An attractive approach for developing novel drugs against TB. Eur. J. Med. Chem. 2016, 124, 160–185. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Kutateladze, A.G. Photoinitiated Cascade for Rapid Access to Pyrroloquinazolinone Core of Vasicinone, Luotonins, and Related Alkaloids. Org. Lett. 2019, 21, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Kucukguzel, I.; Kucukquzel, S.G.; Rollas, S.; Otuk-Sanis, G.; Ozdemir, O.; Bayrak, I.; Altug, T.; Stables, J.P. Synthesis of some 3-(arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. Farmaco 2004, 59, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Sameem, B.; Saeedi, M.; Mahdavi, M.; Shafiee, A. A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 128, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Madhasu, M.; Doda, S.R.; Begari, P.K.; Dasari, K.R.; Thalari, G.; Kadari, S.; Yadav, J.S. Concise total synthesis of antiarrhythmic drug dronedarone via a conjugate addition followed intramolecular heck cyclization. J. Heterocycl. Chem. 2021, 58, 1861–1866. [Google Scholar] [CrossRef]
- Meng, S.; Tang, G.-L.; Pan, H.-X. Enzymatic Formation of Oxygen-Containing Heterocycles in Natural Product Biosynthesis. ChemBioChem 2018, 19, 2002–2022. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, D.; Zhang, Z.-J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y.; Wang, R. One-Pot Enantioselective Synthesis of Functionalized Pyranocoumarins and 2-Amino-4H-chromenes: Discovery of a Type of Potent Antibacterial Agent. J. Org. Chem. 2012, 77, 878–888. [Google Scholar] [CrossRef]
- Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.-X.; et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol. Cancer Ther. 2004, 3, 1375–1384. [Google Scholar]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2020, 212, 113034. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, M.; Das, P. Application of cyclohexane-1,3-diones for six-membered oxygen-containing heterocycles synthesis. Bioorganic Chem. 2020, 107, 104559. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.; Amstutz, R. Natural Compounds as Drugs; Birkhäuser Basel: Basel, Switzerland, 2008; p. 377. [Google Scholar]
- Pathania, S.; Narang, R.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, M.; Torroba, T. Sulfur-Nitrogen Heterocycles. Molecules 2005, 10, 318–320. [Google Scholar] [CrossRef]
- Qian, M.C.; Fan, X.; Mahattanatawee, K. Volatile Sulfur Compounds in Food; Americal Chemical Society: Washington, DC, SUA, 2011; p. 31. [Google Scholar]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar] [CrossRef]
- Demkowicz, S.; Rachon, J.; Daśko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Madhu, M.; Doda, S.R.; Begari, P.K.; Dasari, K.R.; Thalari, G.; Kadari, S.; Yadav, J.S. Enantioselective epoxidation by the chiral auxiliary approach: Asymmetric total synthesis of (+)-Ambrisentan. J. Heterocycl. Chem. 2020, 58, 942–946. [Google Scholar] [CrossRef]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti-infective Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Petraitiene, R.; Petraitis, V.; Maung, B.W.; Naing, E.; Kavaliauskas, P.; Walsh, T.J. Posaconazole Alone and in Combination with Caspofungin for Treatment of Experimental Exserohilum rostratum Meningoencephalitis: Developing New Strategies for Treatment of Phaeohyphomycosis of the Central Nervous System. J. Fungi. 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girmenia, C. New generation azole antifungals in clinical investigation. Expert Opin. Investig. Drugs 2009, 18, 1279–1295. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; Dimopoulos, Christoph Lange on behalf of the European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Schweer, K.E.; Bangard, C.; Hekmat, K.; Cornely, O.A. Chronic pulmonary aspergillosis. Mycoses 2013, 57, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Maertens, J.; De Bel, A.; Nulens, E.; Boelens, J.; Surmont, I.; Mertens, A.; Boel, A.; Lagrou, K. Nationwide Surveillance of Azole Resistance in Aspergillus Diseases. Antimicrob. Agents Chemother. 2015, 59, 4569–4576. [Google Scholar] [CrossRef] [Green Version]
- Valipour, M.; Naderi, N.; Heidarli, E.; Shaki, F.; Motafeghi, F.; Amiri, F.T.; Emami, S.; Irannejad, H. Design, synthesis and biological evaluation of naphthalene-derived (arylalkyl)azoles containing heterocyclic linkers as new anticonvulsants: A comprehensive in silico, in vitro, and in vivo study. Eur. J. Pharm. Sci. 2021, 166, 105974. [Google Scholar] [CrossRef]
- Sui, Y.-F.; Li, D.; Wang, J.; Bheemanaboina, R.R.Y.; Ansari, M.F.; Gan, L.-L.; Zhou, C.-H. Design and biological evaluation of a novel type of potential multi-targeting antimicrobial sulfanilamide hybrids in combination of pyrimidine and azoles. Bioorganic Med. Chem. Lett. 2020, 30, 126982. [Google Scholar] [CrossRef]
- Lv, P.-C.; Sun, J.; Luo, Y.; Yang, Y.; Zhu, H.-L. Design, synthesis, and structure–activity relationships of pyrazole derivatives as potential FabH inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 4657–4660. [Google Scholar] [CrossRef]
- Foroumadi, A.; Mirzaei, M.; Shafiee, A. Antituberculosis agents, I: Synthesis and antituberculosis activity of 2-aryl-1,3,4-thiadiazole derivatives. Die Pharm. 2001, 56, 610–612. [Google Scholar]
- Zhan, P.; Li, D.; Chen, X.; Liu, X.; De Clercq, E. Functional Roles of Azoles Motif in Anti-HIV Agents. Curr. Med. Chem. 2011, 18, 29–46. [Google Scholar] [CrossRef]
- Mermer, A.; Bayrak, H.; Şirin, Y.; Emirik, M.; Demirbaş, N. Synthesis of novel Azol-β-lactam derivatives starting from phenyl piperazine and investigation of their antiurease activity and antioxidant capacity comparing with their molecular docking studies. J. Mol. Struct. 2019, 1189, 279–287. [Google Scholar] [CrossRef]
- Palaska, E.; Şahin, G.; Kelicen, P.; Durlu, N.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. IL Farm. 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Tiperciuc, B.; Pârvu, A.; Tamaian, R.; Nastasă, C.M.; Ionuţ, I.; Oniga, O. New anti-inflammatory thiazolyl-carbonyl-thiosemicarbazides and thiazolyl-azoles with antioxidant properties as potential iNOS inhibitors. Arch. Pharmacal Res. 2013, 36, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Khodairy, A.; Ahmed, E.A.; Ismael, M.; Mohamed, K.M.; Thabet, S.A. Design and Synthesis of Some New Analgesic Azole Derivatives Containing Tramadol Moiety. J. Heterocycl. Chem. 2019, 56, 1055–1062. [Google Scholar] [CrossRef]
- Hou, Y.; Shang, C.; Meng, T.; Lou, W. Anticancer potential of cardiac glycosides and steroid-azole hybrids. Steroids 2021, 171, 108852. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P.K.; Ghosh, K.; Chattopadhyay, S. Synthetic strategies, crystal structures and biological activities of metal complexes with the members of azole family: A review. Polyhedron 2021, 200, 115093. [Google Scholar] [CrossRef]
- Hurtado, J.; Ibarra, L.; Yepes, D.; García-Huertas, P.; Macías, M.A.; Triana-Chavez, O.; Nagles, E.; Suescun, L.; Muñoz-Castro, A. Synthesis, crystal structure, catalytic and anti-Trypanosoma cruzi activity of a new chromium(III) complex containing bis(3,5-dimethylpyrazol-1-yl)methane. J. Mol. Struct. 2017, 1146, 365–372. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Zayed, M.; Abdallah, S. Metal complexes of a novel Schiff base derived from sulphametrole and varelaldehyde. Synthesis, spectral, thermal characterization and biological activity. J. Mol. Struct. 2010, 979, 62–71. [Google Scholar] [CrossRef]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef]
- Castillo, K.F.; Bello, N.; Nuñez-Dallos, N.; Pastrana, H.; Celis, A.M.; Restrepo, S.; Hurtado, J.; Ávila, A.G. Metal Complex Derivatives of Azole: A Study on Their Synthesis, Characterization, and Antibacterial and Antifungal Activities. J. Braz. Chem. Soc. 2016, 27, 2334–2347. [Google Scholar] [CrossRef]
- Gagini, T.; Colina-Vegas, L.; Villarreal, W.; Borba-Santos, L.P.; Pereira, C.D.S.; Batista, A.A.; Fleury, M.K.; de Souza, W.; Rozental, S.; Costa, L.A.S.; et al. Metal–azole fungistatic drug complexes as anti-Sporothrix spp. agents. New J. Chem. 2018, 42, 13641–13650. [Google Scholar] [CrossRef]
- Sharma, M.; Prasher, P. An epigrammatic status of the ‘azole’-based antimalarial drugs. RSC Med. Chem. 2019, 11, 184–211. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Harrypersad, S.; Sahyon, H.A.; Abu El-Magd, M.; Walsby, C.J. Ruthenium(II)/(III) DMSO-Based Complexes of 2-Aminophenyl Benzimidazole with In Vitro and In Vivo Anticancer Activity. Molecules 2020, 25, 4284. [Google Scholar] [CrossRef] [PubMed]
- Sapijanskaitė-Banevič, B.; Palskys, V.; Vaickelionienė, R.; Šiugždaitė, J.; Kavaliauskas, P.; Grybaitė, B.; Mickevičius, V. Synthesis and Antibacterial Activity of New Azole, Diazole and Triazole Derivatives Based on p-Aminobenzoic Acid. Molecules 2021, 26, 2597. [Google Scholar] [CrossRef] [PubMed]
- Grybaitė, B.; Vaickelionienė, R.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Kantminienė, K.; Novikov, V.; Mickevičius, V. Synthesis and Antimicrobial Activity of Novel Thiazoles with Reactive Functional Groups. Chem. Select. 2019, 4, 6965–6970. [Google Scholar] [CrossRef]
- Parašotas, I.; Anusevičius, K.; Vaickelionienė, R.; Jonuškienė, I.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavets, O.; Novikov, V.; Belyakov, S.; Mickevičius, V. Synthesis and evaluation of the antibacterial, antioxidant activities of novel func-tionalized thiazole and bis(thiazol-5-yl)methane derivatives. Arkivoc 2018, 3, 240–256. [Google Scholar] [CrossRef]
- Tumosienė, I.; Peleckis, A.; Jonuškienė, I.; Vaickelionienė, R.; Kantminienė, K.; Šiugždaitė, J.; Beresnevičius, Z.J.; Mickevičius, V. Synthesis of novel 1,2- and 2-substituted benzimidazoles with high antibacterial and antioxidant activity. Mon. Chem. Chem. 2018, 149, 577–594. [Google Scholar] [CrossRef]
- Balandis, B.; Ivanauskaitė, G.; Smirnovienė, J.; Kantminienė, K.; Matulis, D.; Mickevičius, V.; Zubrienė, A. Synthesis and structure–affinity relationship of chlorinated pyrrolidinone-bearing benzenesulfonamides as human carbonic anhydrase inhibitors. Bioorganic Chem. 2020, 97, 103658. [Google Scholar] [CrossRef]
- Beresnevicius, Z.J.; Mickevicius, V.; Rutkauskas, K.; Kantminiene, K. Synthesis of hexahydropyrimidine derivatives and their polymer stabilizing properties. Pol. J. Chem. Technol. 2003, 5, 75–81. [Google Scholar]
- Stasevych, M.; Lubenets, V.; Musyanovych, R.; Novikov, V.; Mickevicius, V.; Beresnevicius, Z.I.; Rutkauskas, K. Novel thi-azolone derivatives of N-aryl-β-alanines. Chem. Heterocycl. Compd. 2011, 47, 1050–1052. [Google Scholar] [CrossRef]
- Kantminienė, K.; Parašotas, I.; Urbonavičiūtė, E.; Anusevičius, K.; Tumosienė, I.; Jonuškienė, I.; Vaickelionienė, R.; Mickevičius, V. Synthesis and Biological Evaluation of Novel Di- and Trisubstituted Thiazole Derivatives. Heterocycles 2017, 94, 1074. [Google Scholar] [CrossRef]
- Tumosienė, I.; Beresnevičius, Z.J.; Kantminienė, K.; Mikulskienė, G. Synthesis of 3-{[2-(N1-alkylidenehydrazinocarbonyl)ethyl](4-alkoxyphenyl)amino}propanohydrazide derivatives and analysis of their isomer composition. Chemija 2008, 19, 44–51. [Google Scholar]
- Daina, A.; Zoete, V. Application of the SwissDrugDesign Online Resources in Virtual Screening. Int. J. Mol. Sci. 2019, 20, 4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavaliauskas, P.; Grybaite, B.; Mickevicius, V.; Petraitiene, R.; Grigaleviciute, R.; Planciuniene, R.; Gialanella, P.; Pockevicius, A.; Petraitis, V. Synthesis, ADMET Properties, and In Vitro Antimicrobial and Antibiofilm Activity of 5-Nitro-2-thiophenecarbaldehyde N-((E)-(5-Nitrothienyl)methylidene)hydrazone (KTU-286) against Staphylococcus aureus with Defined Resistance Mechanisms. Antibiotics 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- De Sá, N.P.; Pôssa, A.P.; Perez, P.; Ferreira, J.M.S.; Fonseca, N.C.; Lino, C.I.; Cruz, L.B.; De Oliveira, R.B.; Rosa, C.A.; Borelli, B.M.; et al. Antifungal Activity Directed Toward the Cell Wall by 2-Cyclohexylidenhydrazo- 4-Phenyl-Thiazole Against Candida albicans. Infect. Disord. Drug Targets. 2019, 19, 428–438. [Google Scholar] [CrossRef]
- Turel, O. Newer antifungal agents. Expert Rev. Anti-Infect. Ther. 2011, 9, 325–338. [Google Scholar] [CrossRef]
- Pricopie, A.-I.; Focșan, M.; Ionuț, I.; Marc, G.; Vlase, L.; Gaina, I.L.; Vodnar, D.C.; Simon, E.; Barta, G.; Pîrnău, A.; et al. Novel 2,4-Disubstituted-1,3-Thiazole Derivatives: Synthesis, Anti-Candida Activity Evaluation and Interaction with Bovine Serum Albumine. Molecules 2020, 25, 1079. [Google Scholar] [CrossRef] [Green Version]
- Jeanvoine, A.; Rocchi, S.; Bellanger, A.; Reboux, G.; Millon, L. Azole-resistant Aspergillus fumigatus: A global phenomenon originating in the environment? Med. Mal. Infect. 2019, 50, 389–395. [Google Scholar] [CrossRef]
- Pérez-Cantero, A.; López-Fernández, L.; Guarro-Artigas, J.; Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents. 2019, 55, 105807. [Google Scholar] [CrossRef]
- Spivak, E.S.; Hanson, K.E. Candida auris: An Emerging Fungal Pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI: Wayne, PA, USA, 2019. [Google Scholar]





| Compound | MW | No of Heavy Atoms | No of Aromatic Heavy Atoms | Fraction Csp3 | Rotatable Bonds | H-Bond Acceptors | H-Bond Donors | Molar Refractivity | TPSA |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 315.39 | 22 | 12 | 0.12 | 7 | 2 | 3 | 92.08 | 110.68 |
| 2a | 433.5 | 31 | 23 | 0.08 | 8 | 4 | 2 | 122.88 | 93.7 |
| 2b | 449.95 | 31 | 23 | 0.08 | 8 | 3 | 2 | 127.93 | 93.7 |
| 2c | 494.4 | 31 | 23 | 0.08 | 8 | 3 | 2 | 130.62 | 93.7 |
| 3 | 463.98 | 32 | 23 | 0.12 | 9 | 3 | 1 | 132.25 | 82.7 |
| 4 | 463.98 | 32 | 23 | 0.08 | 9 | 3 | 3 | 131.87 | 111.52 |
| 5a | 552.09 | 39 | 29 | 0.06 | 11 | 3 | 2 | 162.14 | 97.86 |
| 5b | 570.08 | 40 | 29 | 0.06 | 11 | 4 | 2 | 162.1 | 97.86 |
| 5c | 586.53 | 40 | 29 | 0.06 | 11 | 3 | 2 | 167.15 | 97.86 |
| 5d | 630.99 | 40 | 29 | 0.06 | 11 | 3 | 2 | 169.84 | 97.86 |
| 5e | 597.09 | 42 | 29 | 0.06 | 12 | 5 | 2 | 170.97 | 143.68 |
| 5f | 595.16 | 42 | 29 | 0.12 | 12 | 3 | 2 | 176.35 | 101.1 |
| 5g | 566.12 | 40 | 29 | 0.09 | 11 | 3 | 2 | 167.11 | 97.86 |
| 5h | 558.12 | 38 | 28 | 0.07 | 11 | 3 | 2 | 160.02 | 126.1 |
| 5i | 603.11 | 41 | 28 | 0.07 | 12 | 5 | 2 | 168.84 | 171.92 |
| 5j | 504.05 | 35 | 23 | 0.15 | 10 | 3 | 2 | 147.27 | 97.86 |
| 5k | 518.07 | 36 | 23 | 0.18 | 11 | 3 | 2 | 152.08 | 97.86 |
| 6 | 528.07 | 37 | 28 | 0.14 | 9 | 3 | 1 | 153.08 | 91.29 |
| 7 | 542.09 | 38 | 28 | 0.13 | 10 | 2 | 2 | 158.85 | 90.43 |
| 8 | 599.17 | 41 | 29 | 0.06 | 13 | 2 | 4 | 173.39 | 141.65 |
| 9 | 581.15 | 40 | 34 | 0.06 | 9 | 2 | 2 | 168.15 | 122.1 |
| Comp-ound | Log Kp (cm/s) | GI Absorption | BBB Permeant | P-Gp Substrate | Inhibition of Cytochrome P450 System | ||||
|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | |||||
| 1 | −6.56 | High | No | No | No | No | Yes | No | No |
| 2a | −4.85 | Low | No | No | No | Yes | Yes | Yes | Yes |
| 2b | −4.57 | Low | No | No | No | Yes | Yes | Yes | Yes |
| 2c | −4.8 | Low | No | No | No | Yes | Yes | Yes | Yes |
| 3 | −4.43 | Low | No | Yes | No | Yes | Yes | Yes | Yes |
| 4 | −5.37 | Low | No | No | Yes | Yes | Yes | Yes | Yes |
| 5a | −4.21 | Low | No | No | No | Yes | No | Yes | Yes |
| 5b | −4.25 | Low | No | No | No | Yes | No | Yes | Yes |
| 5c | −3.98 | Low | No | No | No | Yes | No | Yes | Yes |
| 5d | −4.21 | Low | No | No | No | Yes | No | Yes | Yes |
| 5e | −4.61 | Low | No | No | No | Yes | No | No | Yes |
| 5f | −4.39 | Low | No | No | No | Yes | No | Yes | Yes |
| 5g | −4.04 | Low | No | No | No | Yes | No | Yes | Yes |
| 5h | −4.24 | Low | No | No | No | No | Yes | Yes | Yes |
| 5i | −4.4 | Low | No | No | No | Yes | No | No | Yes |
| 5j | −4.94 | Low | No | Yes | No | Yes | Yes | Yes | Yes |
| 5k | −4.7 | Low | No | Yes | No | Yes | Yes | Yes | Yes |
| 6 | −4.25 | Low | No | No | No | Yes | Yes | No | Yes |
| 7 | −4.22 | Low | No | No | No | Yes | No | Yes | Yes |
| 8 | −4.6 | Low | No | No | No | Yes | No | No | Yes |
| 9 | −4.14 | Low | No | No | No | No | No | No | Yes |
| Compound | Gram-Positive | Gram-Negative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S. a MRSA | S. a MSSA | S. a VRSA | E. fec | E. fae VREF | E. raffi | K. pn NDM-1 | S. m R | P. ae AmpC | |
| 1 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 2a | 2 | 2 | 2 | 32 | 32 | 64< | 64< | 64< | 64< |
| 2b | 1 | 1 | 2 | 64< | 64< | 64< | 64< | 64< | 64< |
| 2c | 64 | 32 | 32 | 32 | 32 | 64 | 64< | 64< | 64< |
| 3 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 4 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5a | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5b | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5c | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5d | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5e | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5f | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5g | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5h | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5i | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5j | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5k | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 6 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 7 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 8 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 9 | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| Vancomycin | 2 | 0.5 | 64< | 0.5 | 32 | 32 | n.a | n.a | n.a |
| Daptomycin | 0.5< | 0.5< | 0.5 | 1 | 2 | 2 | n.a | n.a | n.a |
| Meropenem | 4 | 1 | 4 | n.a | n.a | n.a | 32 | 16 | 16 |
| Compound | A. fumigatus Azole Resistance Phenotype | ||
|---|---|---|---|
| L98H, TR34 | F495I, L98H, S297T, TR34 | Wt | |
| 1 | 64< | 64< | 64< |
| 2a | 32 | 32 | 64< |
| 2b | 32 | 32 | 64< |
| 2c | 64 | 64< | 64 |
| 3 | 64< | 64< | 64< |
| 4 | 64< | 64< | 64< |
| 5a | 64< | 64< | 64< |
| 5b | 64< | 64< | 64< |
| 5c | 64< | 64< | 64< |
| 5d | 64< | 64< | 64< |
| 5e | 64< | 64< | 64< |
| 5f | 64< | 64< | 64< |
| 5g | 64< | 64< | 64< |
| 5h | 64< | 64< | 64< |
| 5i | 64< | 64< | 64< |
| 5j | 64< | 64< | 64< |
| 5k | 64< | 64< | 64< |
| 6 | 64< | 64< | 64< |
| 7 | 64< | 64< | 64< |
| 8 | 64< | 64< | 64< |
| 9 | 64< | 64< | 64< |
| Voriconazole | 8 | 1 | 0.5 |
| Itraconazole | 2 | 16< | 1 |
| Compound | C. auris 381 | C. auris 382 | C. auris 383 | C. auris 384 | C. duobushaemulonii 394 | C. krusei 397 | C. albicans 1214 |
|---|---|---|---|---|---|---|---|
| 1 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 2a | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 2b | 32 | 16 | 32 | 32 | 16 | 64< | 64< |
| 2c | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 3 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 4 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5a | 64 | 64 | 64< | 64< | 64< | 32 | 64< |
| 5b | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5c | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5d | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5e | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5f | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5g | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5h | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5i | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5j | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 5k | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 6 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 7 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 8 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| 9 | 64< | 64< | 64< | 64< | 64< | 64< | 64< |
| Fluconazole | 4 | 16 | 64< | 64< | 8 | 64 | 32 |
| Itraconazole | 0.125 | 1 | 0.5 | 0.5 | 0.5 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malūkaitė, D.; Grybaitė, B.; Vaickelionienė, R.; Vaickelionis, G.; Sapijanskaitė-Banevič, B.; Kavaliauskas, P.; Mickevičius, V. Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens. Molecules 2022, 27, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010074
Malūkaitė D, Grybaitė B, Vaickelionienė R, Vaickelionis G, Sapijanskaitė-Banevič B, Kavaliauskas P, Mickevičius V. Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens. Molecules. 2022; 27(1):74. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010074
Chicago/Turabian StyleMalūkaitė, Dovilė, Birutė Grybaitė, Rita Vaickelionienė, Giedrius Vaickelionis, Birutė Sapijanskaitė-Banevič, Povilas Kavaliauskas, and Vytautas Mickevičius. 2022. "Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens" Molecules 27, no. 1: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010074