Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper
Abstract
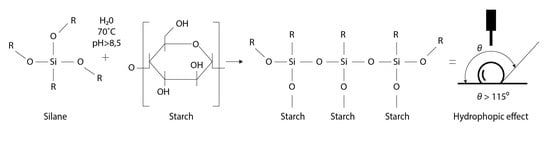
:1. Introduction
2. Results and Discussion
2.1. Water Penetration Dynamics of Modified Starch Applied on a Paper
2.2. Water Repellency Effect of Modified Starch Applied on a Paper
2.3. Effect of the Starch-MTMS Coating on the Selected Strength Properties of Paper Sheets
2.4. Morphology Modification of OMS
3. Materials and Methods
3.1. Preparation of the Starch-Silane Hydrophobic Agent
3.2. Evaluation of Hydrophobic Properties
3.2.1. Water Penetration Dynamics Analysis
3.2.2. Contact Angle Analysis
3.2.3. Water Uptake
3.3. Mechanical Properties of the Paper Samples
3.4. SEM-EDX Analysis of the Modified Starch Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Swinkels, J.J.M. Composition and Properties of Commercial Native Starches. Starch-Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009; ISBN 0-08-092655-X. [Google Scholar]
- Moriana, R.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Improved Thermo-Mechanical Properties by the Addition of Natural Fibres in Starch-Based Sustainable Biocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. In Starch-Based Materials in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 37–76. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Biricik, Y.; Sonmez, S.; Ozden, O. Effects of Surface Sizing with Starch on Physical Strength Properties of Paper. Asian J. Chem. 2011, 23, 3151. [Google Scholar]
- Larotonda, F.D.S.; Matsui, K.N.; Sobral, P.J.A.; Laurindo, J.B. Hygroscopicity and Water Vapor Permeability of Kraft Paper Impregnated with Starch Acetate. J. Food Eng. 2005, 71, 394–402. [Google Scholar] [CrossRef]
- Spiridon, I.; Teacă, C.-A.; Bodîrlău, R.; Bercea, M. Behavior of Cellulose Reinforced Cross-Linked Starch Composite Films Made with Tartaric Acid Modified Starch Microparticles. J. Polym. Environ. 2013, 21, 431–440. [Google Scholar] [CrossRef]
- Molavi, H.; Behfar, S.; Shariati, M.A.; Kaviani, M.; Atarod, S. A Review on Biodegradable Starch Based Film. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 456. [Google Scholar] [CrossRef] [Green Version]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Flexible Starch-Polyurethane Films: Physiochemical Characteristics and Hydrophobicity. Carbohydr. Polym. 2017, 163, 236–246. [Google Scholar] [CrossRef]
- Martinez-Pardo, I.; Shanks, R.A.; Adhikari, B.; Adhikari, R. Thermoplastic Starch-Nanohybrid Films with Polyhedral Oligomeric Silsesquioxane. Carbohydr. Polym. 2017, 173, 170–177. [Google Scholar] [CrossRef]
- Dal, A.B.; Hubbe, M.A. Hydrophobic Copolymers Added with Starch at the Size Press of a Paper Machine: A Review of Findings and Likely Mechanisms. BioResources 2021, 16, 2138. [Google Scholar]
- Donath, S.; Militz, H.; Mai, C. Weathering of Silane Treated Wood. Holz Roh-Und Werkst. 2007, 65, 35. [Google Scholar] [CrossRef]
- Ratajczak, I.; Szentner, K.; Rissmann, I.; Mazela, B.; Hochmanska, P. Treatment Formulation Based on Organosilanes and Plant Oil Blend—Reactivity to Wood and Cellulose. Wood Res. 2012, 57, 265–270. [Google Scholar]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane Coupling Agents Used for Natural Fiber/Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Siuda, J.; Perdoch, W.; Mazela, B.; Zborowska, M. Catalyzed Reaction of Cellulose and Lignin with Methyltrimethoxysilane—FT-IR, 13C NMR and 29Si NMR Studies. Materials 2019, 12, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tshabalala, M.A.; Gangstad, J.E. Accelerated Weathering of Wood Surfaces Coated with Multifunctional Alkoxysilanes by Sol-Gel Deposition. J. Coat. Technol. 2003, 75, 37–43. [Google Scholar] [CrossRef]
- Hill, C.A.; Farahani, M.M.; Hale, M.D. The Use of Organo Alkoxysilane Coupling Agents for Wood Preservation. Holzforschung 2004, 58, 316–325. [Google Scholar] [CrossRef]
- Holik, H. Handbook of Paper and Board; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 3-527-60833-8. [Google Scholar]
- Jonhed, A.; Andersson, C.; Järnström, L. Effects of Film Forming and Hydrophobic Properties of Starches on Surface Sized Packaging Paper. Packag. Technol. Sci. Int. J. 2008, 21, 123–135. [Google Scholar] [CrossRef]
- Hubbe, M.A. Paper’s Resistance to Wetting–A Review of Internal Sizing Chemicals and Their Effects. BioResources 2007, 2, 106–145. [Google Scholar]
- Glittenberg, D.; Becker, A. Cationic Starches for Surface Sizing. Pap. Technol. 1998, 39, 37–41. [Google Scholar]
- Cunha, A.G.; Freire, C.S.; Silvestre, A.J.; Neto, C.P.; Gandini, A. Preparation and Characterization of Novel Highly Omniphobic Cellulose Fibers Organic–Inorganic Hybrid Materials. Carbohydr. Polym. 2010, 80, 1048–1056. [Google Scholar] [CrossRef]
- Paquet, O.; Krouit, M.; Bras, J.; Thielemans, W.; Belgacem, M.N. Surface Modification of Cellulose by PCL Grafts. Acta Mater. 2010, 58, 792–801. [Google Scholar] [CrossRef]
- Cunha, A.G.; Freire, C.; Silvestre, A.; Neto, C.P.; Gandini, A.; Belgacem, M.N.; Chaussy, D.; Beneventi, D. Preparation of Highly Hydrophobic and Lipophobic Cellulose Fibers by a Straightforward Gas–Solid Reaction. J. Colloid Interface Sci. 2010, 344, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Satterly, K.P. Method of Rendering Starch Hydrophobic and Free Flowing. U.S. Patent No. 3,071,492, 1 January 1963. [Google Scholar]
- Amort, J.; Hanisch, H.; Klapdor, U.; van der Maas, H.; Suerken, H.-P. Method for the Modification of Starch in an Aqueous Medium. U.S. Patent No. 4,540,777, 10 September 1985. [Google Scholar]
- Chen, L.; Wang, Y.; Fei, P.; Jin, W.; Xiong, H.; Wang, Z. Enhancing the Performance of Starch-Based Wood Adhesive by Silane Coupling Agent (KH570). Int. J. Biol. Macromol. 2017, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Sun, B.; Zhang, B.; Long, J.; Chen, L.; Tian, Y. Synthesis, Characterization and Hydrophobicity of Silylated Starch Nanocrystal. Carbohydr. Polym. 2016, 136, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; He, L. Synthesis and Properties of Silane-Fluoroacrylate Grafted Starch. Carbohydr. Polym. 2013, 98, 1056–1064. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Chirachanchai, S. Silane Modified Starch for Compatible Reactive Blend with Poly (Lactic Acid). Carbohydr. Polym. 2014, 106, 255–263. [Google Scholar] [CrossRef]
- Sandrine, U.B.; Isabelle, V.; Hoang, M.T.; Chadi, M. Influence of Chemical Modification on Hemp–Starch Concrete. Constr. Build. Mater. 2015, 81, 208–215. [Google Scholar] [CrossRef]
- Ganicz, T.; Olejnik, K.; Rózga-Wijas, K.; Kurjata, J. New Method of Paper Hydrophobization Based on Starch-Cellulose-Siloxane Interactions. BioResources 2020, 15, 4124–4142. [Google Scholar] [CrossRef]
- Ganicz, T.; Rozga-Wijas, K. Siloxane-Starch-Based Hydrophobic Coating for Multiple Recyclable Cellulosic Materials. Materials 2021, 14, 4977. [Google Scholar] [CrossRef]
- Waldner, C.; Hirn, U. Ultrasonic Liquid Penetration Measurement in Thin Sheets—Physical Mechanisms and Interpretation. Materials 2020, 13, 2754. [Google Scholar] [CrossRef]
- Sarah, K.; Ulrich, H. Short Timescale Wetting and Penetration on Porous Sheets Measured with Ultrasound, Direct Absorption and Contact Angle. RSC Adv. 2018, 8, 12861–12869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolpak, F.J.; Weih, M.; Blackwell, J. Mercerization of Cellulose: 1. Determination of the Structure of Mercerized Cotton. Polymer 1978, 19, 123–131. [Google Scholar] [CrossRef]
- Okano, T.; Sarko, A. Mercerization of Cellulose. II. Alkali–Cellulose Intermediates and a Possible Mercerization Mechanism. J. Appl. Polym. Sci. 1985, 30, 325–332. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Wimmer, T.; Schnabel, T.; Petutschnigg, A. Starch-Sugar Synergy in Wood Adhesion Science: Basic Studies and Particleboard Production. Eur. J. Wood Wood Prod. 2012, 70, 271–278. [Google Scholar] [CrossRef]
- Grüner, G. Emtec Penetration-Dynamics Analyzer; Emtec Electronic GmbH Materials: Leipzig, Germany, 1996. [Google Scholar]










| Reference Paper (Uncoated) | Paper Coated with Starch | Paper Coated with Starch + AATMS | Paper Coated with Starch + NTES | Paper Coated with Starch + TES | Paper Coated with Starch + MTMS | |
|---|---|---|---|---|---|---|
| MAX | 0.0 | 0.0800 | 0.0780 | 0.0777 | 0.0777 | 0.4003 |
| t95 | 0.0 | 0.0817 | 0.1837 | 0.2583 | 0.1537 | 1.3593 |
| ΔI/Δt (0.2 s) | 253.7 | 303.1 | 13.7 | 18.6 | 11.2 | −9.6 |
| ΔI/Δt (3 s) | 1.6 | 3.9 | 36.3 | 9.8 | 30.4 | 5.6 |
| Water uptake (Cobb60), g/m2 | 154.5 | 135.8 | 133.5 | 119.1 | 128.2 | 21.5 |
| Acronym | Name | CAS Number | Chemical Structure |
|---|---|---|---|
| MTMS | methyltrimethoxysilane | 1185-55-3 |  |
| NTES | n-octyltriethoxysilane | 2943-75-1 |  |
| AATMS | N-(2-Aminoethyl)-3-aminopropyltrimethoxy silane | 1760-24-3 |  |
| TES | Tetraethoxysilane (Tetraethyl orthosilicate) | 78-10-4 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, T.; Mazela, B.; Olejnik, K.; Peplińska, B.; Perdoch, W. Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper. Molecules 2022, 27, 3136. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103136
Nowak T, Mazela B, Olejnik K, Peplińska B, Perdoch W. Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper. Molecules. 2022; 27(10):3136. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103136
Chicago/Turabian StyleNowak, Tomasz, Bartłomiej Mazela, Konrad Olejnik, Barbara Peplińska, and Waldemar Perdoch. 2022. "Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper" Molecules 27, no. 10: 3136. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103136