Mass Spectrometry-Based Metabolomics of Phytocannabinoids from Non-Cannabis Plant Origins
Abstract
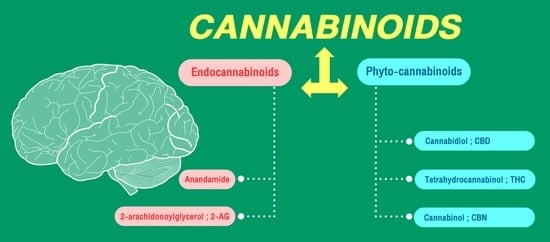
:1. Introduction
2. Endocannabinoid System
3. Mass Spectrometry-Based for Discovery of Phytocannabinoids
4. Phytocannabinoids from Non-Cannabis Plant Origins
5. Non-Cannabis Sources
5.1. Rhododendron Species (Ericaceae Family)
5.2. Other Angiosperm Species
5.3. Liverworts (Radulaceae Family)
6. Legal Consumption
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vásquez-Ocmín, P.G.; Marti, G.; Bonhomme, M.; Mathis, F.; Fournier, S.; Bertani, S.; Maciuk, A. Cannabinoids vs. whole metabolome: Relevance of cannabinomics in analyzing Cannabis varieties. Anal. Chim. Acta 2021, 1184, 339020. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis Sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.C.; Frei, P.; Weber, S.; Scheurer, E.; Mercer-Chalmers-Bender, K. Beyond Δ9-tetrahydrocannabinol and cannabidiol: Chemical differentiation of cannabis varieties applying targeted and untargeted analysis. Anal. Bioanal. Chem. 2022, 414, 3847–3862. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids; Springer: Cham, Switzerland, 2017; pp. 1–36. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Mahlberg, P.G.; Kim, E.S. Accumulation of cannabinoids in glandular trichomes of Cannabis (Cannabaceae). J. Ind. Hemp 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L.D. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Fride, E. Endocannabinoids in the central nervous system—An overview. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommano, S.R.; Tangpao, T.; Pankasemsuk, T.; Ponpanumas, V.; Phimolsiripol, Y.; Rachtanapun, P.; Prasad, S.K. Growing ganja permission: A real gate-way for Thailand’s promising industrial crop? J. Cannabis Res. 2022, 4, 10. [Google Scholar] [CrossRef]
- Ballotta, D.; Bergeron, H.; Hughes, B. Cannabis Control in Europe; EMCDDA Monographs; EMCDDA: Lisbon, Portugal, 2008; p. 99. [Google Scholar]
- Decorte, T.; Pardal, M.; Queirolo, R.; Boidi, M.F.; Avilés, C.S.; Franquero, Ò.P. Regulating Cannabis Social Clubs: A comparative analysis of legal and self-regulatory practices in Spain, Belgium and Uruguay. Int. J. Drug Policy 2017, 43, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of Metabolomics in Cannabis (Cannabis sativa L.) Research and Development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Labar, G.; Wouters, J.; Lambert, D.M. A review on the monoacylglycerol lipase: At the interface between fat and endocannabinoid signalling. Curr. Med. Chem. 2010, 17, 2588–2607. [Google Scholar] [CrossRef] [Green Version]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB (1). Cell 2016, 167, 750–762.e714. [Google Scholar] [CrossRef] [Green Version]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Rosati, O.; Curini, M.; Marcotullio, M.C. Cannabis and Bioactive Cannabinoids. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 17–57. [Google Scholar]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Dettmer, K.; Hammock, B.D. Metabolomics—A new exciting field within the ‘omics’ sciences. Environ. Health Perspect. 2004, 112, A396–A397. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Schlatter, J. Synthetic Cannabinoids: Synthesis and Biological Activities. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 291–311. [Google Scholar]
- Stefkov, G.; Cvetkovikj Karanfilova, I.; Stoilkovska Gjorgievska, V.; Trajkovska, A.; Geskovski, N.; Karapandzova, M.; Kulevanova, S. Analytical Techniques for Phytocannabinoid Profiling of Cannabis and Cannabis-Based Products—A Comprehensive Review. Molecules 2022, 27, 975. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cannazza, G.; Catani, M.; Cavaliere, C.; Cavazzini, A.; Cerrato, A.; Citti, C.; Felletti, S.; Montone, C.M.; Piovesana, S.; et al. Recent applications of mass spectrometry for the characterization of cannabis and hemp phytocannabinoids: From targeted to untargeted analysis. J. Chromatogr. A 2021, 1655, 462492. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. Phytocannabinoids 2017, 103, 61–101. [Google Scholar] [CrossRef]
- Thomas, B.F.; ElSohly, M.A. Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents. In The Analytical Chemistry of Cannabis; Thomas, B.F., ElSohly, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–41. [Google Scholar] [CrossRef]
- Battista, N.; Sergi, M.; Montesano, C.; Napoletano, S.; Compagnone, D.; Maccarrone, M. Analytical approaches for the determination of phytocannabinoids and endocannabinoids in human matrices. Drug Test. Anal. 2014, 6, 7–16. [Google Scholar] [CrossRef]
- Verstraete, A.; Goessaert, A.-S.; Veramme, J. Comparison of the drug concentrations in oral fluid collected by two sampling methods (Varian OraLab and Statsure Saliva Sampler). Ann. Toxicol. Anal. 2011, 23, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Quintela, O.; Andrenyak, D.M.; Hoggan, A.M.; Crouch, D.J. A validated method for the detection of Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in oral fluid samples by liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Anal. Toxicol. 2007, 31, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, F.T.; Remane, D. Aspects of matrix effects in applications of liquid chromatography–mass spectrometry to forensic and clinical toxicology—A review. Anal. Bioanal. Chem. 2012, 403, 2155–2172. [Google Scholar] [CrossRef] [PubMed]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In Marijuana and the Cannabinoids; Springer: Berlin, Germany, 2007; pp. 17–49. [Google Scholar]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytocannabinoids Biosynthesis in Angiosperms, Fungi, and Liverworts and Their Versatile Role. Plants 2021, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.; Fenclova, M.; Peukertova, P.; Binova, Z.; Dzuman, Z.; Hajslova, J. Determination of Seventeen Phytocannabinoids in Various Matrices by UHPLC-HRMS/MS. LC GC Eur. 2020, 33, 8–16. [Google Scholar]
- Citti, C.; Russo, F.; Sgrò, S.; Gallo, A.; Zanotto, A.; Forni, F.; Vandelli, M.A.; Laganà, A.; Montone, C.M.; Gigli, G.; et al. Pitfalls in the analysis of phytocannabinoids in cannabis inflorescence. Anal. Bioanal. Chem. 2020, 412, 4009–4022. [Google Scholar] [CrossRef]
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2017, 123, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.-G.; Hong, K.; Tang, M.; Tang, J.; Liu, L.-X.; Gao, G.-F.; Shen, Z.-J.; Zhang, X.-M.; Yi, Y. Untargeted metabolite profiling of petal blight in field-grown Rhododendron agastum using GC-TOF-MS and UHPLC-QTOF-MS/MS. Phytochemistry 2021, 184, 112655. [Google Scholar] [CrossRef]
- COL. Rhododendron L. In Catalogue of Life; Species 2000 Secretariat: Leiden, The Netherlands, 2022. [Google Scholar]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef]
- Shi, Q.; Li, T.-T.; Wu, Y.-M.; Sun, X.-Y.; Lei, C.; Li, J.-Y.; Hou, A.-J. Meroterpenoids with diverse structures and anti-inflammatory activities from Rhododendron anthopogonoides. Phytochemistry 2020, 180, 112524. [Google Scholar] [CrossRef] [PubMed]
- Hakeem Said, I.; Rezk, A.; Hussain, I.; Grimbs, A.; Shrestha, A.; Schepker, H.; Brix, K.; Ullrich, M.S.; Kuhnert, N. Metabolome Comparison of Bioactive and Inactive Rhododendron Extracts and Identification of an Antibacterial Cannabinoid(s) from Rhododendron collettianum. Phytochem. Anal. 2017, 28, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Kitanaka, S. New Cannabinoid-Like Chromane and Chromene Derivatives from Rhododendron anthopogonoides. Chem. Pharm. Bull. 2011, 59, 1409–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhnert, N.; Hakeem, I.; Said, A.S.; Rezk, A.; Grimbs, A.; Nolzen, J.; Schepker, H.; Brix, K.; Albach, D.; Ullrich, M. Rhododendron Natural Products as Sources of Novel Antibiotics. Rhododendr. Int. 2019, 3, 141–151. [Google Scholar]
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300922. [Google Scholar] [CrossRef] [Green Version]
- Kashiwada, Y.; Yamazaki, K.; Ikeshiro, Y.; Yamagishi, T.; Fujioka, T.; Mihashi, K.; Mizuki, K.; Cosentino, L.M.; Fowke, K.; Morris-Natschke, S.L.; et al. Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron dauricum. Tetrahedron 2001, 57, 1559–1563. [Google Scholar] [CrossRef]
- Taura, F.; Iijima, M.; Kurosaki, F. Daurichromenic acid and grifolic acid: Phytotoxic meroterpenoids that induce cell death in cell culture of their producer Rhododendron dauricum. Plant Signal. Behav. 2018, 13, e1422463. [Google Scholar] [CrossRef] [Green Version]
- Taura, F.; Iijima, M.; Lee, J.-B.; Hashimoto, T.; Asakawa, Y.; Kurosaki, F. Daurichromenic Acid-producing Oxidocyclase in the Young Leaves of Rhododendron dauricum. Nat. Prod. Commun. 2014, 9, 1329–1332. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-X.; Wang, J.-X.; Wang, Q.; Li, H.-L.; Tao, M.; Luo, Q.; Liu, H. New chromane and chromene meroterpenoids from flowers of Rhododendron rubiginosum Franch. var. rubiginosum. Fitoterapia 2018, 127, 396–401. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Guo, P.-J.; Wang, X.-L.; Chen, H.-M.; Chen, L.-J.; Sang, Y.-L.; Hao, Y.-J.; Lu, J. Study on phytochemical and pharmacological activities of four Rhododendron plants endemic to Northeast China. J. Agric. Food Res. 2022, 7, 100255. [Google Scholar] [CrossRef]
- Liao, H.-B.; Huang, G.-H.; Yu, M.-H.; Lei, C.; Hou, A.-J. Five Pairs of Meroterpenoid Enantiomers from Rhododendron capitatum. J. Org. Chem. 2017, 82, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Kjaerulff, L.; Hansen, P.R.; Kongstad, K.T.; Staerk, D. Dual High-Resolution α-Glucosidase and PTP1B Inhibition Profiling Combined with HPLC-PDA-HRMS-SPE-NMR Analysis for the Identification of Potentially Antidiabetic Chromene Meroterpenoids from Rhododendron capitatum. J. Nat. Prod. 2021, 84, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-B.; Lei, C.; Gao, L.-X.; Li, J.-Y.; Li, J.; Hou, A.-J. Two Enantiomeric Pairs of Meroterpenoids from Rhododendron capitatum. Org. Lett. 2015, 17, 5040–5043. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, S.; De Leo, M.; Cervelli, C.; Ruffoni, B.; Ciccarelli, D.; Pistelli, L. Essential Oil Composition and Volatile Profile of Seven Helichrysum Species Grown in Italy. Chem. Biodivers. 2018, 15, e1700545. [Google Scholar] [CrossRef]
- Najar, B.; Pieracci, Y.; Cervelli, C.; Flamini, G.; Pistelli, L. Volatolomics of Three South African Helichrysum Species Grown in Pot under Protected Environment. Molecules 2021, 26, 7283. [Google Scholar] [CrossRef]
- Sauer, S. Amorfrutins: A Promising Class of Natural Products that Are Beneficial to Health. ChemBioChem 2014, 15, 1231–1238. [Google Scholar] [CrossRef]
- Curtis, B.J.; Micikas, R.J.; Burkhardt, R.N.; Smith, R.A.; Pan, J.Y.; Jander, K.; Schroeder, F.C. Syntheses of Amorfrutins and Derivatives via Tandem Diels–Alder and Anionic Cascade Approaches. J. Org. Chem. 2021, 86, 11269–11276. [Google Scholar] [CrossRef]
- Han, J.; Heo, H.; Jeong, M.; Kim, H.; Jang, I. Review on amorfrutin of licorice for type2 diabetes mellitus. J. Intern. Korean Med. 2020, 41, 1078–1088. [Google Scholar] [CrossRef]
- Kulma, A.; Skórkowska-Telichowska, K.; Kostyn, K.; Szatkowski, M.; Skała, J.; Drulis-Kawa, Z.; Preisner, M.; Żuk, M.; Szperlik, J.; Wang, Y.F.; et al. New flax producing bioplastic fibers for medical purposes. Ind. Crops Prod. 2015, 68, 80–89. [Google Scholar] [CrossRef]
- Hussain, T.; Plunkett, B.; Ejaz, M.; Espley, R.V.; Kayser, O. Identification of Putative Precursor Genes for the Biosynthesis of Cannabinoid-Like Compound in Radula marginata. Front. Plant Sci. 2018, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Styrczewska, M.; Kulma, A.; Ratajczak, K.; Amarowicz, R.; Szopa, J. Cannabinoid-like anti-inflammatory compounds from flax fiber. Cell Mol. Biol. Lett. 2012, 17, 479–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Espley, R.V.; Gertsch, J.; Whare, T.; Stehle, F.; Kayser, O. Demystifying the liverwort Radula marginata, a critical review on its taxonomy, genetics, cannabinoid phytochemistry and pharmacology. Phytochem. Rev. 2019, 18, 953–965. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Asakawa, Y. Terpenoids and aromatic compounds from Bryophytes and their central nervous system activity. Curr. Org. Chem. 2020, 24, 113–128. [Google Scholar] [CrossRef]
- Chicca, A.; Schafroth, M.A.; Reynoso-Moreno, I.; Erni, R.; Petrucci, V.; Carreira, E.M.; Gertsch, J. Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high. Sci. Adv. 2018, 4, eaat2166. [Google Scholar] [CrossRef] [Green Version]
- Toyota, M.; Shimamura, T.; Ishii, H.; Renner, M.; Braggins, J.; Asakawa, Y. New bibenzyl cannabinoid from the New Zealand liverwort Radula marginata. Chem. Pharm. Bull. 2002, 50, 1390–1392. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Dey, A. The ethno-medicinal and pharmaceutical attributes of Bryophytes: A review. Phytomed. Plus 2022, 2, 100255. [Google Scholar] [CrossRef]
- Burstein, S.H. Eicosanoid mediation of cannabinoid actions. Bioorg. Med. Chem. 2019, 27, 2718–2728. [Google Scholar] [CrossRef]
- Cullmann, F.; Becker, H. Prenylated Bibenzyls from the Liverwort Radula laxiramea. Z. Nat. C 1999, 54, 147–150. [Google Scholar] [CrossRef]
- Lancione, S.; Wade, K.; Windle, S.B.; Filion, K.B.; Thombs, B.D.; Eisenberg, M.J. Non-medical cannabis in North America: An overview of regulatory approaches. Public Health 2020, 178, 7–14. [Google Scholar] [CrossRef]
- De Aquino, J.P.; Sherif, M.; Radhakrishnan, R.; Cahill, J.D.; Ranganathan, M.; D’Souza, D.C. The Psychiatric Consequences of Cannabinoids. Clin. Ther. 2018, 40, 1448–1456. [Google Scholar] [CrossRef] [Green Version]
- Haroutounian, S.; Gilron, I.; Belton, J.; Degenhardt, L.; Di Forti, M.; Finn, D.P.; Fogarty, A.; Kalso, E.; Krane, E.; Moore, R.A.; et al. Societal issues and policy implications related to the use of cannabinoids, cannabis, and cannabis-based medicines for pain management. Pain 2021, 162, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Ware, M.; Müller-Vahl, K.; Abrams, D.; Grotenhermen, F. The Medicinal Use of Cannabis and Cannabinoids—An International Cross-Sectional Survey on Administration Forms. J. Psychoact. Drugs 2013, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2017, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef]



| Compound Classes | Chemical Structure | Number of Compounds * | Therapeutic Activity |
|---|---|---|---|
| Δ9-trans-tetrahydrocannabinol |  | 9 subclasses Δ9 tetrahydrocannabinol and its derivatives; R1 = C5H11, R2 = H Δ9 tetrahydrocannabinolic acid B; R1 = C5H11, R2 = COOH Δ9 tetrahydrocannabinol-C4 and its derivatives; R1 = C4H9, R2 = H or COOH Δ9 tetrahydrocannabivarin and its derivatives; R1 = C3H7, C2 = H Δ9 tetrahydrocannabiorcol and its derivatives; R1 = CH3, R2 = H or COOH | Euphoriant, analgesic, anti-inflammatory, antioxidant, antiemetic |
| Δ8-trans-tetrahydrocannabinol |  | 2 subclasses Δ8 tetrahydrocannabinol and its derivatives; R1 = COOH, R2 = C5H11 | Euphoriant, analgesic, anti-inflammatory, antioxidant, antiemetic |
| Cannabidiol |  | 7 subclasses Cannabidiol and its derivatives; R1 = C5H11; R2 = H Cannabidivarin and its derivatives; R1 = C3H7, R2 = H | Antibiotic, antixiolytic, antiphychotic, analgesic, antioxidant, antispasmodic, anti-inflammatory |
| Cannabigerol |  | 6 subclasses Cannabigerol and its derivatives; R1 = C5H11, R2 = H Cannabigerol monomethyl ether and its derivatives; R1 = C5H11, R2 = CH3 Cannabigerovarin forms; R1 = C3H7, R2 = H | Antibiotic, antifungal, anti-inflammatory, analgesic |
| Cannabichromene |  | 4 subclasses Cannabichromeme and its derivatives; R1 = C5H11, R2 = CH3 Cannabichromevarin and its derivatives; R1 = C3H7, R2 = CH3 | Antibiotic, antifungal, anti-inflammatory, analgesic |
| Cannabinol |  | 9 subclasses Cannabinol and its derivatives; R = H, R1 = H or COOH, R2 = C5H11 Cannabivarin; R = H, R1 = H, R2 = C3H7 Cannabiorcol; R = H, R1 = H, R2 = C2H5 Cannabinodiol; R = C5H11 Cannabinodivarin; R = C3H7 | Sedative, antibiotic anticonvulsant, anti-inflammatory |
| Cannabinodiol |  | ||
| Cannabicyclol |  | 3 subclasses Cannabicyclol and derivatives; R1 = C5H11 Cannabicyclovarin; R1 = C3H7 | |
| Cannabielsoin |  | 3 subclasses Cannabielsoin and its derivatives; R1 = C5H11, R2 = H or COOH | |
| Cannabitriol |  | 5 subclasses Cannabitriol; R = H, R1 = OH, R2 = C5H11 10-ethoxy-9-hydroxy Δ6a- tetrahydrocannabinol; R = H, R1 = OC2H5, R2 = C5H11 8,9-hydroxy Δ6a- tetrahydrocannabinol; R = OH, R1 = H, R2 = C5H11 Cannabitroolvarin; R=H, R1 = OH, R2 = C3H7 Ethoxy-cannabitriolvarin; R = H, R1 = OC2H5, R2 = C3H7 | |
| Unclassified types |  | - For examples; de-hydrocannabifuran DCBF-C5, cannabi-furan CBF-C5- |
| Rhododendron spp. | Plant Part Used for Extraction | Mass Spectrometry-Based Metabolomics | Novel or Specific PhytocannaBinoid Identified |
|---|---|---|---|
R.anthopogonoides | twigs and leaves [48,49] | HR-FAB-MS with UV, IR and 1H-NMR spectrums [50] HR-ESI-MS couple with 1D NMR, and HSQC spectra [48] |  Anthopogochromenic acid  Chromene derivative (anthopogocyclolic acid) |
R. dauricum L. | Leaf | molecular formula was established by HR-FAB-MS coupling with 1H-NMR and 13C spectra [53]. |  Daurichromenic acid  Rhododaurichromanic acid A  Rhododaurichromanic acid B |
R. rubiginosum | Flower | molecular formula was established by HR-ESI-MS, couple with IR, UV 1D and 2D NMR spectras. The planer structure was estab-lished by its 1H-1H COSY and HMBC spectra [56]. |  Rubiginosin and its derivatives |
R. capitatum | Aerial part | HR-MS with solid phase extraction coupled with 1H and 13C NMR [59]. |  Capitachromenic acid A and its derivatives |
Everlasting, Helichrysum umbraculigerum | Licorice, Glycyrrhiza foetida | Bastard indigobush, Amorpha fruticose | Flax fibre, Linum usitatissimum  |
 Cannabigerol and its derivatives  Amorfrutin A and its derivatives |  Amorfrutin A  Amorfrutin B | Non-characterised compound | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommano, S.R.; Sunanta, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Sringarm, K.; Ruksiriwanich, W.; Jantrawut, P.; et al. Mass Spectrometry-Based Metabolomics of Phytocannabinoids from Non-Cannabis Plant Origins. Molecules 2022, 27, 3301. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103301
Sommano SR, Sunanta P, Leksawasdi N, Jantanasakulwong K, Rachtanapun P, Seesuriyachan P, Phimolsiripol Y, Sringarm K, Ruksiriwanich W, Jantrawut P, et al. Mass Spectrometry-Based Metabolomics of Phytocannabinoids from Non-Cannabis Plant Origins. Molecules. 2022; 27(10):3301. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103301
Chicago/Turabian StyleSommano, Sarana Rose, Piyachat Sunanta, Noppol Leksawasdi, Kittisak Jantanasakulwong, Pornchai Rachtanapun, Phisit Seesuriyachan, Yuthana Phimolsiripol, Korawan Sringarm, Warintorn Ruksiriwanich, Pensak Jantrawut, and et al. 2022. "Mass Spectrometry-Based Metabolomics of Phytocannabinoids from Non-Cannabis Plant Origins" Molecules 27, no. 10: 3301. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103301