Light-Responsive Hexagonal Assemblies of Triangular Azo Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Size Control of Hexagonal Structures
2.2. Growth into Fluorescent Hexagonal Nanorods
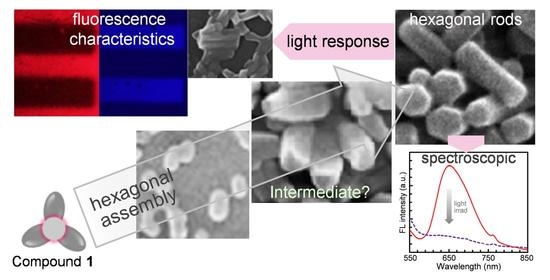
2.3. Light-Induced Hexagonal-to-Amorphous Transition
2.4. Hexagonal-to-Amorphous Transition Accompanied by Prominent Changes in Fluorescence Characteristics and Good Contrast of Microscopic Images
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Compound 1
4.2. Materials
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Liao, Q.; Yao, J. Construction and Optoelectronic Properties of Organic One-Dimensional Nanostructures. Acc. Chem. Res. 2010, 43, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, T.; Lee, M. Responsive Nanostructures from Aqueous Assembly of Rigid−Flexible Block Molecules. Acc. Chem. Res. 2011, 44, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Jiang, H.; Zou, G.; Zhang, Q. Photo-responsive cholesterol-substituted diacetylenic organogels: Morphology tuning, photo-switching and photo-polymerization. Soft Matter 2013, 9, 9785–9791. [Google Scholar] [CrossRef]
- Abendroth, J.M.; Bushuyev, O.S.; Weiss, P.S.; Barrett, C.J. Controlling Motion at the Nanoscale: Rise of the Molecular Machines. ACS Nano 2015, 9, 7746–7768. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, P.; Wu, B.; Xing, Y.; Shi, K.; Fang, W.; Yu, H.; Wang, G. Photochromic Dendrimers for Photoswitched Solid-To-Liquid Transitions and Solar Thermal Fuels. ACS Appl. Mater. Interfaces 2020, 12, 50135–50142. [Google Scholar] [CrossRef]
- Herbert, K.M.; Schrettl, S.; Rowan, S.J.; Weder, C. 50th Anniversary Perspective: Solid-State Multistimuli, Multiresponsive Polymeric Materials. Macromolecules 2017, 50, 8845–8870. [Google Scholar] [CrossRef]
- Harris, J.D.; Moran, M.J.; Aprahamian, I. New Molecular Switch Architectures. Proc. Natl. Acad. Sci. USA 2018, 115, 9414–9422. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Liu, B.; Liu, J.; Wei, P.; Zhang, H.; Han, T.; Qi, J.; Lam, J.W.Y.; Zhang, W.; Tang, B.Z. “Seeing” and Controlling Photoisomerization by (Z)-/(E)-Isomers with Aggregation-Induced Emission Characteristics. ACS Nano 2019, 13, 12120–12126. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Wang, Y.; Li, Z. Supramolecular Self-Assembly of Perylene Bisimide Derivatives Assisted by Various Groups. Langmuir 2019, 35, 342–358. [Google Scholar] [CrossRef]
- Yuan, Y.; He, L.; Li, J.; Zhang, H. Synthesis, properties and photo-responsive behavior of luminescent side chain polymers containing D–π-A α-cyanostilbene units. Polym. Chem. 2019, 10, 2706–2715. [Google Scholar] [CrossRef]
- Abe, I.; Hara, M.; Seki, T.; Cho, S.J.; Shimizu, M.; Matsuura, K.; Cheong, H.; Kim, J.Y.; Oh, J.; Jung, J.; et al. A trigonal molecular assembly system with the dual light-driven functions of phase transition and fluorescence switching. J. Mater. Chem. C 2019, 7, 2276–2282. [Google Scholar] [CrossRef]
- Han, T.; Yao, Z.; Qiu, Z.; Zhao, Z.; Wu, K.; Wang, J.; Poon, A.W.; Lam, J.W.Y.; Tang, B.Z. Photoresponsive spiro-polymers generated in situ by C–H-activated polyspiroannulation. Nat. Commun. 2019, 10, 5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Aggarwal, H.; Srivastava, A. Of Twists and Curves: Electronics, Photophysics, and Upcoming Applications of Non-Planar Conjugated Organic Molecules. Chem. Eur. J. 2020, 26, 10653–10675. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Kim, Y.; Lee, M. Supramolecular Chiral 2D Materials and Emerging Functions. Adv. Mater. 2020, 32, 1905669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Guo, S.; Liu, W.; Yang, Q.; Sun, H.; Ding, R.; Qian, Z.; Feng, H. Rational design of reversibly photochromic molecules with aggregation-induced emission by introducing photoactive thienyl and benzothienyl groups. J. Mater. Chem. C 2020, 8, 13197–13204. [Google Scholar] [CrossRef]
- Luo, W.; Wang, G. Photo-Responsive Fluorescent Materials with Aggregation-Induced Emission Characteristics. Adv. Opt. Mater. 2020, 8, 2001362. [Google Scholar] [CrossRef]
- Gayathri, P.; Pannipara, M.; Al-Sehemi, A.; Anthony, S.P. Triphenylamine-based stimuli-responsive solid state fluorescent materials. New J. Chem. 2020, 44, 8680–8696. [Google Scholar] [CrossRef]
- Krishnamohan Sharma, C.V.; Clearfield, A. Three-Dimensional Hexagonal Structures from a Novel Self-Complementary Molecular Building Block. J. Am. Chem. Soc. 2000, 122, 4394–4402. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, Y.; Yang, W.; Yao, J.; Zhu, L.; Shuai, Z. Low-Dimensional Aggregates from Stilbazolium-Like Dyes. Angew. Chem. Int. Ed. 2004, 43, 4060–4063. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Srivastava, S.; Kotov, N.A. Nanoparticle assembly for 1D and 2D ordered structures. Soft Matter 2009, 5, 1146–1156. [Google Scholar] [CrossRef]
- Gunderson, V.L.; Mickley Conron, S.M.; Wasielewski, M.R. Self-assembly of a hexagonal supramolecular light-harvesting array from chlorophyll a trefoil building blocks. Chem. Commun. 2010, 46, 401–403. [Google Scholar] [CrossRef]
- de Rooy, S.L.; El-Zahab, B.; Li, M.; Das, S.; Broering, E.; Chandler, L.; Warner, I.M. Fluorescent One-Dimensional Nanostructures from a Group of Uniform Materials Based on Organic Salts. Chem. Commun. 2011, 47, 8916. [Google Scholar] [CrossRef] [PubMed]
- Danila, I.; Riobé, F.; Piron, F.; Puigmartí-Luis, J.; Wallis, J.D.; Linares, M.; Ågren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical Chiral Expression from the Nano- to Mesoscale in Synthetic Supramolecular Helical Fibers of a Nonamphiphilic C3-Symmetrical π-Functional Molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Cantekin, S.; Greef, T.F.A.; Palmans, A.R.A. Benzene-1,3,5-Tricarboxamide: A Versatile Ordering Moiety for Supramo-lecular Chemistry. Chem. Soc. Rev. 2012, 41, 6125–6137. [Google Scholar] [CrossRef]
- Kim, Y.; Li, W.; Shin, S.; Lee, M. Development of Toroidal Nanostructures by Self-Assembly: Rational Designs and Applications. Acc. Chem. Res. 2013, 46, 2888–2897. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kageyama, H.; Oaki, Y.; Imai, H. Direction Control of Oriented Self-Assembly for 1D, 2D, and 3D Microarrays of Anisotropic Rectangular Nanoblocks. J. Am. Chem. Soc. 2014, 136, 3716–3719. [Google Scholar] [CrossRef]
- Anuradha; La, D.D.; Al Kobaisi, M.; Bhosale, S.V. Right handed chiral superstructures from achiral molecules: Self-assembly with a twist. Sci. Rep. 2015, 5, 15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, K.J.M. Hierarchical Self-Assembly for Nanomedicine. Angew. Chem. Int. Ed. 2016, 55, 1598–1600. [Google Scholar] [CrossRef]
- Lewandowska, U.; Zajaczkowski, W.; Pisula, W.; Ma, Y.; Li, C.; Müllen, K.; Wennemers, H. Effect of Structural Modifications on the Self-Assembly of Oligoprolines Conjugated with Sterically Demanding Chromophores. Chem. Eur. J. 2016, 22, 3804–3809. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Chen, J.; Liu, M. Hierarchical Self-Assembly of a Porphyrin into Chiral Macroscopic Flowers with Superhydrophobic and Enantioselective Property. ACS Nano 2017, 11, 12453–12460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, K.; Liljeroth, P. Molecular assembly on two-dimensional materials. Nanotechnology 2017, 28, 082001. [Google Scholar] [CrossRef] [PubMed]
- Kajjam, A.B.; Giri, S.; Vaidyanathan, S. Triphenylamine-based donor–π–acceptor organic phosphors: Synthesis, characterization and theoretical study. Mater. Chem. Front. 2017, 1, 512–520. [Google Scholar] [CrossRef]
- Tothadi, S.; Koner, K.; Dey, K.; Addicoat, M.; Banerjee, R. Morphological Evolution of Two-Dimensional Porous Hexagonal Trimesic Acid Framework. ACS Appl. Mater. Interfaces 2020, 12, 15588–15594. [Google Scholar] [CrossRef]
- Shimizu, T.; Ding, W.; Kameta, N. Soft-Matter Nanotubes: A Platform for Diverse Functions and Applications. Chem. Rev. 2020, 120, 2347–2407. [Google Scholar] [CrossRef]
- Nadimetla, D.N.; Al Kobaisi, M.; Bugde, S.T.; Bhosale, S.V. Tuning Achiral to Chiral Supramolecular Helical Superstructures. Chem. Rec. 2020, 20, 793–819. [Google Scholar] [CrossRef]
- Messina, G.M.L.; Mazzuca, C.; Dettin, M.; Zamuner, A.; Di Napoli, B.; Ripani, G.; Marletta, G.; Palleschi, A. From nanoaggregates to mesoscale ribbons: The multistep self-organization of amphiphilic peptides. Nanoscale Adv. 2021, 3, 3605–3614. [Google Scholar] [CrossRef]
- Forber, C.L.; Kelusky, E.C.; Bunce, N.J.; Zerner, M.C. Electronic spectra of cis- and trans-azobenzenes: Consequences of ortho substitution. J. Am. Chem. Soc. 1985, 107, 5884–5890. [Google Scholar] [CrossRef]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene Photoswitching without Ultraviolet Light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef]
- Bunce, N.J.; Ferguson, G.; Forber, C.L.; Stachnyk, G.J. Sterically Hindered Azobenzenes: Isolation of Cis Isomers and Kinetics of Thermal Cis→Trans Isomerization. J. Org. Chem. 1987, 52, 394–398. [Google Scholar] [CrossRef]
- Han, M.R.; Hashizume, D.; Hara, M. 1-[(E)-3-sec-Butyl-4-(4′-ethoxy-3,5-diethylbiphenyl-4-yldiazenyl)phenoxy]hexadecane. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3001–o3003. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Ishikawa, D.; Honda, T.; Ito, E.; Hara, M. Light-driven molecular switches in azobenzene self-assembled monolayers: Effect of molecular structure on reversible photoisomerization and stable cis state. Chem. Commun. 2010, 46, 3598. [Google Scholar] [CrossRef] [PubMed]
- Hirade, T.; Okui, Y.; Han, M. A design strategy for stable light-sensitive palladium complexes. J. Mater. Chem. C 2013, 1, 2672–2679. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Abe, I.; Han, M. Green-light-induced melting of self-assembled azobenzene nano/microstructures. New J. Chem. 2019, 43, 19014–19019. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Budzak, S.; Simeth, N.A.; Hansen, M.J.; Feringa, B.L.; Jacquemin, D.; Szymanski, W. General Principles for the Design of Visible-Light-Responsive Photoswitches: Tetra-ortho-Chloro-Azobenzenes. Angew. Chem. Int. Ed. 2020, 59, 21663–21670. [Google Scholar] [CrossRef]
- Rau, H. Photochromism, Molecules and Systems; Dürr, H., Buuas-Laurent, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Sekkat, Z.; Knoll, W. Photoreactive Organic Thin Films in the Light of Bound Electromagnetic Waves; John and Wiley and Sons: Hoboken, NJ, USA, 1997; pp. 117–195. [Google Scholar]
- Rau, H. Spectroscopic properties of organic azo compounds, Angew. Chem. Int. Ed. Engl. 1973, 12, 224. [Google Scholar] [CrossRef]
- Morgante, C.G.; Struve, W.S. S2→S0 fluorescence in trans-azobenzene. Chem. Phys. Lett. 1979, 68, 267–271. [Google Scholar] [CrossRef]
- Azuma, J.; Tamai, N.; Shishido, A.; Ikeda, T. Femtosecond dynamics and stimulated emission from the S2 state of a liquid crystalline trans-azobenzene. Chem. Phys. Lett. 1998, 288, 77–82. [Google Scholar] [CrossRef]
- Satzger, H.; Spörlein, S.; Root, C.; Wachtveitl, J.; Zinth, W.; Gilch, P. Fluorescence spectra of trans- and cis-azobenzene—emission from the Franck–Condon state. Chem. Phys. Lett. 2003, 372, 216–223. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Synthesis of the most intensely fluorescent azobenzene by utilizing the B–N interaction. Chem. Commun. 2007, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Furuta, A.; Kambe, T.; Itoi, H.; Kano, N.; Kawashima, T.; Ito, Y.; Asashima, M. Intensely Fluorescent Azobenzenes: Synthesis, Crystal Structures, Effects of Substituents, and Application to Fluorescent Vital Stain. Chem. Eur. J. 2010, 16, 4951. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Fluorescent azobenzenes and aromatic aldimines featuring an N–B interaction. Dalton Trans. 2013, 42, 15826. [Google Scholar] [CrossRef] [Green Version]
- Gabor, G.; Frei, Y.; Gegiou, D.; Kaganowitch, M.; Fischer, E. Tautomerism and Geometric Isomerism in Arylazo-Phenols and Naphthols. Part III. Ortho-hydroxy Derivatives and their Reversible Photochemical Reactions. Isr. J. Chem. 1967, 5, 193–211. [Google Scholar] [CrossRef]
- Ghasemian, M.; Kakanejadifard, A.; Azarbani, F.; Zabardasti, A.; Kakanejadifard, S. Spectroscopy and solvatochromism studies along with antioxidant and antibacterial activities investigation of azo–azomethine compounds 2-(2-hydroxyphenylimino)methyl)-4-phenyldiazenyl)phenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 124, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Guan, P.; Fang, W. Photoinduced Proton Transfer and Isomerization in a Hydrogen-Bonded Aromatic Azo Compound: A CASPT2//CASSCF Study. J. Phys. Chem. A 2014, 118, 4732–4739. [Google Scholar] [CrossRef]
- Racané, L.; Mihalić, Z.; Cerić, H.; Popović, J.; Tralić-Kulenović, V. Synthesis, structure and tautomerism of two benzothiazolyl azo derivatives of 2-naphthol: A crystallographic, NMR and computational study. Dyes Pigm. 2013, 96, 672–678. [Google Scholar] [CrossRef]
- Yoon, Y.; Jo, S.; Park, S.J.; Kim, H.M.; Kim, D.; Lee, T.S. Unusual fluorescence of o-phenylazonaphthol derivatives with aggregation-induced emission and their use in two-photon cell imaging. Chem. Commun. 2019, 55, 6747–6750. [Google Scholar] [CrossRef]
- Lee, H.Y.; Song, X.; Park, H.; Baik, M.; Lee, D. Torsionally responsive C3-symmetric azo dyes: Azo-hydrazone tautomerism, conformational switching, and application for chemical sensing. J. Am. Chem. Soc. 2010, 132, 12133–12144. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- An, B.; Kwon, S.; Jung, S.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-Naphthalimide AIE/AIEEgens: Recent Advances and Prospects. ACS Appl. Mater. Interfaces 2018, 10, 12081–12111. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-Induced Emission-Active Gels: Fabrications, Functions, and Applications. Adv. Mater. 2021, 33, 2100021. [Google Scholar] [CrossRef]
- Tokumura, Y.; Han, M. Petal-like microstructures formed from sterically crowded chromophores. J. Photopolym. Sci. Technol. 2021, 34, 417–421. [Google Scholar] [CrossRef]
- Han, M.; Cho, S.J.; Norikane, Y.; Shimizu, M.; Kimura, A.; Tamagawa, T.; Seki, T. Multistimuli-responsive azobenzene nanofibers with aggregation-induced emission enhancement characteristics. Chem. Commun. 2014, 50, 15815–15818. [Google Scholar] [CrossRef]
- Han, M.; Abe, I.; Matsuura, K.; Takeoka, Y.; Seki, T. Morphologically diverse micro- and macrostructures created via solvent evaporation-induced assembly of fluorescent spherical particles in the presence of polyethylene glycol derivatives. Molecules 2021, 26, 4294. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yokoyama, K.; Aratani, N.; Yamada, H.; Masuo, S. Crystallization-Induced Emission of Azobenzene Deriva-tives. Angew. Chem. Int. Ed. 2019, 58, 14173–14178. [Google Scholar] [CrossRef]
- Kathiravan, A.; Sundaravel, K.; Jaccob, M.; Dhinagaran, G.; Rameshkumar, A.; Ananth, D.A.; Sivasudha, T. Pyrene Schiff Base: Photophysics, Aggregation Induced Emission, and Antimicrobial Properties. J. Phys. Chem. B 2014, 118, 13573–13581. [Google Scholar] [CrossRef]
- Gascón-Moya, M.; Pejoan, A.; Izquierdo-Serra, M.; Pittolo, S.; Cabré, G.; Hernando, J.; Alibés, R.; Gorostiza, P.; Busqué, F. An Optimized Glutamate Receptor Photoswitch with Sensitized Azobenzene Isomerization. J. Org. Chem. 2015, 80, 9915–9925. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Abe, I.; Oh, J.; Jung, J.; Son, Y.J.; Noh, J.; Hara, M.; Seki, T. Solvent- and Light-Sensitive AIEE-Active Azo Dye: From Spherical to 1D and 2D Assemblies. Int. J. Mol. Sci. 2022, 23, 965. [Google Scholar] [CrossRef]
- Iijima, S.; Ichibashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603. [Google Scholar] [CrossRef]
- Davis, R.; Rath, N.P.; Das, S. Thermally reversible fluorescent polymorphs of alkoxy-cyano-substituted diphenylbutadienes: Role of crystal packing in solid state fluorescence. Chem. Commun. 2004, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. Self-Assembled Hexa- peri -hexabenzocoronene Graphitic Nanotube. Science 2004, 304, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- Mena-Osteritz, E. Superstructures of Self-Organizing Thiophenes. Adv. Mater. 2002, 14, 609–616. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsutsumi, O. Optical switching and image storage by means of azobenzene liquid-crystal films. Science 1995, 268, 1873–1875. [Google Scholar] [CrossRef]
- Yu, Y.; Ikeda, T. Photocontrollable Liquid-Crystalline Actuators. Adv. Mater. 2011, 23, 2149–2180. [Google Scholar] [CrossRef]
- Norikane, Y.; Hirai, Y.; Yoshida, M. Photoinduced isothermal phase transitions of liquid-crystalline macrocyclic azobenzenes. Chem. Commun. 2011, 47, 1770. [Google Scholar] [CrossRef]
- Uchida, E.; Azumi, R.; Norikane, Y. Light-induced crawling of crystals on a glass surface. Nature Commun. 2015, 6, 7310. [Google Scholar] [CrossRef]
- Yue, Y.; Norikane, Y.; Azumi, R.; Koyama, E. Light-induced mechanical response in crosslinked liquid-crystalline polymers with photoswitchable glass transition temperatures. Nature Commun. 2018, 9, 3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Moe, K. Light-Responsive Hexagonal Assemblies of Triangular Azo Dyes. Molecules 2022, 27, 4380. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144380
Han M, Moe K. Light-Responsive Hexagonal Assemblies of Triangular Azo Dyes. Molecules. 2022; 27(14):4380. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144380
Chicago/Turabian StyleHan, Mina, and Khin Moe. 2022. "Light-Responsive Hexagonal Assemblies of Triangular Azo Dyes" Molecules 27, no. 14: 4380. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144380