First Organic–Inorganic Hybrid Compounds Formed by Ge-V-O Clusters and Transition Metal Complexes of Aromatic Organic Ligands
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Data Analysis
2.2. Syntheses of Compounds Based on Ge-V-O Clusters
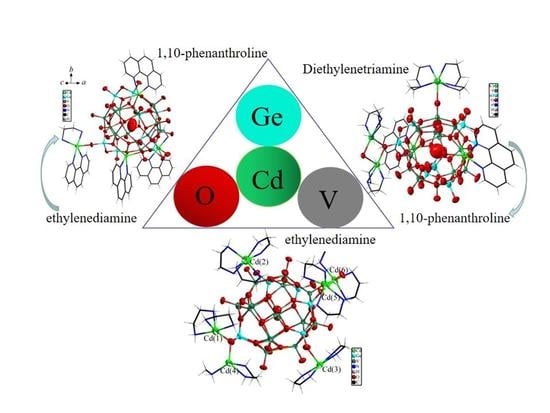
2.2.1. [Cd(phen)(en)]2[Cd2(phen)2V12O40Ge8(OH)8(H2O)]∙12.5H2O (1)
2.2.2. [Cd(DETA)]2[Cd(DETA)2]0.5[Cd2(phen)2V12O41Ge8(OH)7(0.5H2O)]∙7.5H2O (2)
2.2.3. [Cd(en)3]{[Cd(η2-en)2]3[Cd(η2-en)(η1-en)(η2-en)Cd][Ge6V15O48(H2O)]}∙5.5H2O (3)
2.3. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis Description
3.2. Description of Crystal Structures
3.2.1. [Cd(phen)(en)]2[Cd2(phen)2V12O40Ge8(OH)8(H2O)]∙12.5H2O (1)
3.2.2. Cd(DETA)]2[Cd(DETA)2]0.5[Cd2(phen)2V12O41Ge8(OH)7(0.5H2O)]∙7.5H2O (2)
3.2.3. [Cd(en)3]{[Cd(η2-en)2]3[Cd(η2-en)(η2-μ2-en)(η2-en)Cd][Ge6V15O48(H2O)]}∙5.5H2O (3)
3.3. BVS Calculations to Determine the Locations of Hydrogen Atoms of Compounds 1–3
3.4. IR Spectrophotometry
3.5. XRD Powder Diffractometer
3.6. UV-Vis Spectrophotometry
3.7. ESR Spectrophotometry
3.8. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Colloid. Interface. Sci. 2020, 281, 102178. [Google Scholar] [CrossRef]
- Vidyavathi, G.T.; Kumar, B.V.; Raghu, A.V.; Aravinda, T.; Hani, U.; Ananda-Murthy, H.C.; Shridhar, A.H. Punica granatum pericarp extract catalyzed green chemistry approach for synthesizing novel ligand and its metal(II) complexes: Molecular docking/DNA interactions. J. Mol. Struct. 2022, 1249, 131656. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, K.R.; Reddy, C.V.; Shetti, N.P.; Sadhu, V.; Shankar, M.V.; Reddy, V.G.; Raghu, A.V.; Aminabhavi, T.M. Nanostructured Materials for Environmental Applications; Balakumar, S., Keller, V., Shankar, M.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; p. 485. [Google Scholar]
- Zhou, J.; Zhang, J.; Fang, W.H.; Yang, G.Y. A Series of Vanadogermanates from 1D Chain to 3D Framework Built by Ge–V–O Clusters and Transition-Metal-Complex Bridges. Chem. Eur. J. 2010, 16, 13253. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Yamaguchi, S.; Nakagawa, Y.; Uehara, K.; Uchida, S.; Yamaguchi, K.; Mizuno, N. Synthesis of a Dialuminum-Substituted Silicotungstate and the Diastereoselective Cyclization of Citronellal Derivatives. J. Am. Chem. Soc. 2008, 130, 15872–15878. [Google Scholar] [CrossRef]
- Nyman, M.; Bonhomme, F.; Alam, T.M.; Bodriguez, M.A.; Cherry, B.R.; Krumhansl, J.L.; Nenoff, T.M.; Sattler, A.M. A General Synthetic Procedure for Heteropolyniobates. Science 2002, 297, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Kögerler, P.; Cronin, L. Polyoxometalate Nanostructures, Superclusters, and Colloids: From Functional Clusters to Chemical Aesthetics. Angew. Chem. Int. Ed. 2005, 44, 844–846. [Google Scholar] [CrossRef]
- Rüther, T.; Hultgren, V.M.; Timko, B.P.; Bond, A.M.; Jackson, W.R.; Wedd, A.G. Electrochemical Investigation of Photooxidation Processes Promoted by Sulfo-polyoxometalates: Coupling of Photochemical and Electrochemical Processes into an Effective Catalytic Cycle. J. Am. Chem. Soc. 2003, 125, 10133–10143. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, J.; Yang, G.Y. Designed Synthesis of POM–Organic Frameworks from {Ni6PW9} Building Blocks under Hydrothermal Conditions. Angew. Chem. Int. Ed. Engl. 2008, 47, 3909–3913. [Google Scholar] [CrossRef]
- Zheng, S.T.; Yuan, D.Q.; Jia, H.P.; Zhang, J.; Yang, G.Y. Combination between lacunary polyoxometalates and high-nuclear transition metal clusters under hydrothermal conditions: I. from isolated cluster to 1-D chain. Chem. Commun. 2007, 18, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Q.; Li, Y.G.; Zhang, Z.M.; Qin, C.; Wang, X.L.; Wang, E.B.; Su, Z.M. Chiral Polyoxometalate-Induced Enantiomerically 3D Architectures: A New Route for Synthesis of High-Dimensional Chiral Compounds. J. Am. Chem. Soc. 2007, 129, 10066–10067. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.I.; Streb, C.; Thiel, J.; Mitchell, S.G.; Miras, H.N.; Long, D.L.; Boyd, T.; Peacock, R.D.; McGlone, T.; Cronin, L. Reversible Redox Reactions in an Extended Polyoxometalate Framework Solid. Angew. Chem. Int. Ed. 2008, 47, 6881–6884. [Google Scholar] [CrossRef] [PubMed]
- Streb, C.; Ritchie, C.; Long, D.L.; Kögerler, P.; Cronin, L. Modular Assembly of a Functional Polyoxometalate-Based Open Framework Constructed from Unsupported AgI⋅⋅⋅AgI Interactions. Angew. Chem. Int. Ed. 2007, 46, 7579–7582. [Google Scholar] [CrossRef] [PubMed]
- Long, D.L.; Streb, C.; Song, Y.F.; Mitchell, S.; Cronin, L. Unravelling the Complexities of Polyoxometalates in Solution Using Mass Spectrometry: Protonation versus Heteroatom Inclusion. J. Am. Chem. Soc. 2008, 130, 1830–1832. [Google Scholar] [CrossRef]
- Yoshida, A.; Nakagawa, Y.; Uehara, K.; Hikichi, S.; Mizuno, N. Inorganic Cryptand: Size-Selective Strong Metallic Cation Encapsulation by a Disilicoicosatungstate (Si2W20) Polyoxometalate. Angew. Chem. Int. Ed. 2009, 48, 7055–7058. [Google Scholar] [CrossRef]
- Micoine, K.; Hasenknopf, B.; Thorimbert, S.; Lacote, E.; Malacria, M. Chiral Recognition of Hybrid Metal Oxide by Peptides. Angew. Chem. Int. Ed. 2009, 48, 3466–3468. [Google Scholar] [CrossRef]
- Müller, A.; Fedin, V.P.; Kuhlmann, C.; Fenske, H.D.; Baum, G.; Bögge, H.; Hauptfleisch, B. ‘Adding’ stable functional complementary, nucleophilic and electrophilic clusters: A synthetic route to [{(SiW11O39)Mo3S4(H2O)3(µ-OH)}2]10− and [{(P2W17O61)Mo3S4(H2O)3(µ-OH)}2]14− as examples. Chem. Commun. 1999, 35, 1189–1190. [Google Scholar] [CrossRef]
- Knaust, J.M.; Inman, C.; Keller, S.W. A host–guest complex between a metal–organic cyclotriveratrylene analog and a polyoxometalate: [Cu6(4,7-phenanthroline)8(MeCN)4]2PM12O40 (M = Mo or W). Chem. Commun. 2004, 40, 492–493. [Google Scholar] [CrossRef]
- Ishii, Y.; Takenaka, Y.; Konishi, K. Porous Organic–Inorganic Assemblies Constructed from Keggin Polyoxometalate Anions and Calix[4]arene–Na+ Complexes: Structures and Guest-Sorption Profiles. Angew. Chem. Int. Ed. 2004, 43, 2702–2705. [Google Scholar] [CrossRef]
- Wei, M.L.; He, C.; Sun, Q.Z.; Meng, Q.J.; Duan, C.Y. Zeolite Ionic Crystals Assembled through Direct Incorporation of Polyoxometalate Clusters within 3D Metal–Organic Frameworks. Inorg. Chem. 2007, 46, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Wu, Q.; Wang, J.P. 1D and 2D polyoxometalate-based composite compounds. Synthesis and crystal structure of [{Ba(DMSO)5(H2O)}2(SiMo12O40)] and [{Ba(DMSO)3(H2O)3}{Ba(DMSO)5(H2O)}(GeMo12O40)]. J. Chem. Soc. Dalton Trans. 2002, 31, 2512–2516. [Google Scholar] [CrossRef]
- Sha, J.Q.; Peng, J.; Tian, A.X.; Liu, H.S.; Chen, J.; Zhang, P.P.; Su, Z.M. Assembly of Multitrack Cu–N Coordination Polymeric Chain-Modified Polyoxometalates Influenced by Polyoxoanion Cluster and Ligand. Cryst. Growth Des. 2007, 7, 2535–2541. [Google Scholar] [CrossRef]
- Cui, X.B.; Sun, Y.Q.; Yang, G.Y. Hydrothermal synthesis and characterization of a novel two-dimensional framework materials constructed from the polyoxometalates and coordination groups: [As8IIIV14IVO42(CO3)][Cu(en)2]3∙10H2O. Inorg. Chem. Commun. 2003, 6, 259–261. [Google Scholar] [CrossRef]
- Cui, X.B.; Xu, J.Q.; Li, Y.; Sun, Y.H.; Yang, G.Y. Hydrothermal Synthesis and Characterization of a Novel Sinusoidal Layer Structure Constructed from Polyoxometalates and Coordination Complex Fragments. Eur. J. Inorg. Chem. 2004, 7, 1051–1055. [Google Scholar] [CrossRef]
- Cui, X.B.; Xu, J.Q.; Sun, Y.H.; Li, Y.; Ye, L.; Yang, G.Y. Hydrothermal synthesis and crystal structure of a novel 1-D chain structure constructed from polyoxometalates and coordination complex fragments. Inorg. Chem. Commun. 2004, 7, 58–61. [Google Scholar] [CrossRef]
- Guo, H.Y.; Li, Z.F.; Zhao, D.C.; Hu, Y.Y.; Xiao, L.N.; Cui, X.B.; Guan, J.Q.; Xu, J.Q. The synthesis and characterization of three organic–inorganic hybrids based on different transition metal complexes and {As8V14O42(H2O)} clusters. CrystEngComm 2014, 16, 2251–2259. [Google Scholar] [CrossRef]
- Guo, H.Y.; Li, Z.F.; Fu, L.W.; Hu, Y.Y.; Cui, X.B.; Liu, B.J.; Xu, J.N.; Huo, Q.S.; Xu, J.Q. Structural influences of arsenic–vanadium clusters and transition metal complexes on final structures of arsenic–vanadium-based hybrids. Inorg. Chim. Acta 2016, 443, 118–125. [Google Scholar] [CrossRef]
- Guo, H.Y.; Li, Z.F.; Zhang, X.; Fu, L.W.; Hu, Y.Y.; Guo, L.L.; Cui, X.B.; Huo, Q.S.; Xu, J.Q. New self-assembly hybrid compounds based on arsenic–vanadium clusters and transition metal mixed-organic-ligand complexes. CrystEngComm 2016, 18, 566–579. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, J.; Xu, J.Q.; Yang, G.Y. Hybrid inorganic–organic 1-D and 2-D frameworks with {As8V14O42} clusters as building blocks. J. Solid State Chem. 2005, 178, 3740–3746. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, J.; Li, B.; Yang, G.Y. The first solid composed of {As4V16O42(H2O)} clusters. Dalton Trans. 2008, 41, 5584–5587. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.T.; Chen, Y.M.; Zhang, J.; Xu, J.Q.; Yang, G.Y. Hybrid Inorganic–Organic 1D and 2D Frameworks with [As6V15O42]6− Polyoxoanions as Building Blocks. Eur. J. Inorg. Chem. 2006, 9, 397–406. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, M.; Yang, G.Y. [{Zn(enMe)2}2(enMe)2{Zn2As8V12O40(H2O)}]∙4H2O: A Hybrid Molecular Material Based on Covalently Linked Inorganic Zn–As–V Clusters and Transition Metal Complexes via enMe Ligands. Eur. J. Inorg. Chem. 2004, 2004–2007. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, S.T.; Fang, W.H.; Yang, G.Y. A New 2-D Network Containing {As4V16O42(H2O)} Cluster Units. Eur. J. Inorg. Chem. 2009, 34, 5057. [Google Scholar]
- Qi, Y.F.; Li, Y.G.; Wang, E.B.; Jin, H.; Zhang, Z.M.; Wang, X.L.; Chang, S. Syntheses and structures of four organic–inorganic hybrids based on the surface restricted building unit and various zinc coordination groups. Inorg. Chim. Acta 2007, 360, 1841–1853. [Google Scholar] [CrossRef]
- Qi, Y.F.; Li, Y.G.; Wang, E.B.; Jin, H.; Zhang, Z.M.; Wang, X.L.; Chang, S. Two unprecedented inorganic–organic boxlike and chainlike hybrids based on arsenic–vanadium clusters linked by nickel complexes. J. Solid State Chem. 2007, 180, 382–389. [Google Scholar] [CrossRef]
- Qi, Y.F.; Li, Y.G.; Qin, C.; Wang, E.B.; Jin, H.; Xiao, D.R.; Wang, X.L.; Chang, S. From Chain to Network: Design and Analysis of Novel Organic–Inorganic Assemblies from Organically Functionalized Zinc-Substituted Polyoxovanadates and Zinc Organoamine Subunits. Inorg. Chem. 2007, 46, 3217–3230. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhao, X.L.; Xu, J.Q.; Wang, T.G. A novel two-dimensional structure containing the first antimony-substituted polyoxovandium clusters: [{Co(en)2}2SbIII8VIV14O42(H2O)]∙6H2O. J. Chem. Soc. Dalton Trans. 2002, 31, 3275–3276. [Google Scholar] [CrossRef]
- Hu, X.X.; Xu, J.Q.; Cui, X.B.; Song, J.F.; Wang, T.G. A novel one-dimensional framework material constructed from antimony-substituted polyoxovanadium clusters: [(C2N2H10)2β-{SbIII8VIV14O42(H2O)}](C2N2H8)∙4H2O. Inorg. Chem. Commun. 2004, 7, 264. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, X.; Cui, X.B.; Huo, Q.S.; Xu, J.Q. Vanadoantimonates: From discrete clusters to high dimensional aggregates. CrystEngComm 2016, 18, 5130–5139. [Google Scholar] [CrossRef]
- Antonova, E.; Näther, C.; Kögerler, P.; Bensch, W. Organic Functionalization of Polyoxovanadates: Sb-N Bonds and Charge Control. Angew. Chem. Int. Ed. 2011, 50, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Näther, C.; Kögerler, P.; Bensch, W. A C2-symmetric antimonato polyoxovanadate cluster [V16Sb4O42(H2O)]8− derived from the {V18O42} archetype. Dalton Trans. 2012, 41, 6957–6962. [Google Scholar] [CrossRef] [Green Version]
- Antonova, E.; Näther, C.; Bensch, W. Antimonato polyoxovanadates with structure directing transition metal complexes: Pseudopolymorphic {Ni(dien)2}3[V15Sb6O42(H2O)]∙nH2O compounds and {Ni(dien)2}4[V16Sb4O42(H2O)]. Dalton Trans. 2012, 41, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Näther, C.; Bensch, W. Assembly of [V15Sb6O42(H2O)]6− cluster shells into higher dimensional aggregates via weak Sb⋯N/Sb⋯O intercluster interactions and a new polyoxovanadate with a discrete [V16Sb4O42(H2O)]8− cluster shell. CrystEngComm 2012, 14, 6853–6859. [Google Scholar] [CrossRef]
- Wutkowski, A.; Näther, C.; Kögerler, P.; Bensch, W. [V16Sb4O42(H2O){VO(C6H14N2)2}4]: A Terminal Expansion to a Polyoxovanadate Archetype. Inorg. Chem. 2008, 47, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Wutkowski, A.; Näther, C.; Kögerler, P.; Bensch, W. Antimonato Polyoxovanadate Based Three-Dimensional Framework Exhibiting Ferromagnetic Exchange Interactions: Synthesis, Structural Characterization, and Magnetic Investigation of {[Fe(C6H14N2)2]3[V15Sb6O42(H2O)]}∙8H2O. Inorg. Chem. 2013, 52, 3280–3284. [Google Scholar] [CrossRef]
- Kiebach, R.; Näther, C.; Bensch, W. [C6H17N3]4[Sb4V16O42]⋅2H2O and [NH4]4[Sb8V14O42]⋅2H2O—The first isolated Sb derivates of the [V18O42] family. Solid. State. Sci. 2006, 8, 964–970. [Google Scholar] [CrossRef]
- Kiebach, R.; Näther, C.; Kögerler, P.; Bensch, W. [VIV15SbIII6O42]6−: An antimony analogue of the molecular magnet [V15As6O42(H2O)]6−. Dalton Trans. 2007, 36, 3221–3223. [Google Scholar] [CrossRef]
- Lühmann, H.; Näther, C.; Kögerler, P.; Bensch, W. Solvothermal synthesis and crystal structure of a heterometal-bridged {V15Sb6} dimer: [Ni2(tren)3(V15Sb6O42(H2O)0.5)]2[Ni(trenH)2]∙H2O. Inorg. Chim. Acta 2014, 421, 549–552. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.P.; Wang, J.P.; Niu, J.Y. Hydrothermal Synthesis and Crystal Structure of a New 2D Layer Compound [{Ni(en)2}2Sb8V14O42]∙5.5H2O. Chem. Res. Chin. Univ. 2009, 25, 426–429. [Google Scholar]
- Gao, Y.Z.; Han, Z.G.; Xu, Y.Q.; Hu, C.W. pH-Dependent Assembly of Two Novel Organic–inorganic Hybrids Based on Vanadoantimonate Clusters. J. Cluster. Sci. 2010, 21, 163–171. [Google Scholar] [CrossRef]
- Monakhov, K.Y.; Bensch, W.; Kögerler, P. Semimetal-functionalised polyoxovanadates. Chem. Soc. Rev. 2015, 44, 8443–8483. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.B.; Xu, J.Q.; Meng, H.; Zheng, S.T.; Yang, G.Y. A Novel Chainlike As–V–O Polymer Based on a Transition Metal Complex and a Dimeric Polyoxoanion. Inorg. Chem. 2004, 43, 8005–8009. [Google Scholar] [CrossRef] [PubMed]
- You, L.S.; Zhu, Q.Y.; Zhang, X.; Pu, Y.Y.; Bian, G.Q.; Dai, J. A new type of germanium–vanadate cluster, [Ge5V6O21(heda)6] (Hheda = N-(2-hydroxyethyl)ethylenediamine). CrystEngComm 2013, 15, 2411–2415. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, E.B.; Lin, B.Z.; Wang, S.T. The first polyoxoalkoxovanadium germanate anion with a novel cage-like structure: Solvothermal synthesis and characterization. Dalton Trans. 2003, 32, 519–520. [Google Scholar] [CrossRef]
- Whitfield, T.; Wang, X.; Jacobson, A.J. Vanadogermanate Cluster Anions. Inorg. Chem. 2003, 42, 3728–3733. [Google Scholar] [CrossRef]
- Bi, L.H.; Kortz, U.; Dickman, M.H.; Nellutla, S.; Dalal, N.S.; Keita, B.; Nadjo, L.; Prinz, M.; Neumann, M. Polyoxoanion with Octahedral Germanium(IV) Hetero Atom: Synthesis, Structure, Magnetism, EPR, Electrochemistry and XPS Studies on the Mixed-Valence 14-Vanadogermanate [GeVV12VIV2O40]8−. J. Cluster Sci. 2006, 17, 143–165. [Google Scholar] [CrossRef]
- Wang, J.; Näther, C.; Kögerler, P.; Bensch, W. Synthesis, crystal structure and magnetism of a new mixed germanium–polyoxovanadate cluster. Inorg. Chim. Acta 2010, 363, 4399. [Google Scholar] [CrossRef]
- Tripathi, A.; Hughbanks, T.; Clearfield, A. The First Framework Solid Composed of Vanadosilicate Clusters. J. Am. Chem. Soc. 2003, 125, 10528–10529. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, D.; Wang, J.; Hoffmann, R.D.; Pöttgen, R.; Bensch, W. Vanadoantimonates: From discrete clusters to high dimensional aggregates Two Compounds Containing the Mixed Germanium–Vanadium Polyoxothioanion [V14Ge8O42S8]12−. Angew. Chem. Int. Ed. 2006, 45, 1305–1308. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Xu, Y.Q.; Li, S.; Han, Z.G.; Cao, Y.; Cui, F.Y.; Hu, C.W. Syntheses, structures, and magnetism of {V15M6O42(OH)6(Cl)} (M=Si, Ge). J. Coord. Chem. 2010, 63, 3373–3383. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Xu, Y.Q.; Huang, K.L.; Han, Z.G.; Hu, C.W. Two three-dimensional {V16Ge4}− based open frameworks stabilized by diverse types of CoII-amine bridges and magnetic properties. Dalton Trans. 2012, 41, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Näther, C.; Speldrich, M.; Kögerler, P.; Bensch, W. Chain and layer networks of germanato-polyoxovanadates. CrystEngComm 2013, 15, 10283. [Google Scholar] [CrossRef]
- Wang, J.; Näther, C.; Kögerler, P.; Bensch, W. [V15Ge6O42S6(H2O)]12−, a Thiogermanatopolyoxovanadate Cluster Featuring the Spin Topology of the Molecular Magnet [V15As6O42(H2O)]6−. Eur. J. Inorg. Chem. 2012, 1237–1242. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.W.; Wei, Q.; Zhang, J.; Yang, G.Y. Two Tetra-CdII-Substituted Vanadogermanate Frameworks. J. Am. Chem. Soc. 2014, 136, 5065–5071. [Google Scholar] [CrossRef]
- Gao, G.G.; Li, F.Y.; Xu, L.; Liu, X.Z.; Yang, Y.Y. CO2 Coordination by Inorganic Polyoxoanion in Water. J. Am. Chem. Soc. 2008, 130, 10838–10839. [Google Scholar] [CrossRef]
- Xiao, F.P.; Hao, J.; Zhang, J.; Lv, C.L.; Yin, P.C.; Wang, L.S.; Wei, Y.G. Polyoxometalatocyclophanes: Controlled Assembly of Polyoxometalate-Based Chiral Metallamacrocycles from Achiral Building Blocks. J. Am. Chem. Soc. 2010, 132, 5956–5957. [Google Scholar] [CrossRef]
- Miras, H.N.; Wilson, E.F.; Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 2009, 1297–1311. [Google Scholar] [CrossRef]
- Sha, J.Q.; Peng, J.; Liu, H.S.; Chen, J.; Tian, A.X.; Zhang, P.P. Asymmetrical Polar Modification of a Bivanadium-Capped Keggin POM by Multiple Cu−N Coordination Polymeric Chains. Inorg. Chem. 2007, 46, 11183–11189. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466. [Google Scholar] [CrossRef]
- Singh, C.; Mukhopadhyay, S.; Das, S.K. Polyoxometalate-Supported Bis(2,2′-bipyridine)mono(aqua)nickel(II) Coordination Complex: An Efficient Electrocatalyst for Water Oxidation. Inorg. Chem. 2018, 57, 6479–6490. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sec. A 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagrman, P.J.; Zubieta, J. Structural Influences of Organonitrogen Ligands on Vanadium Oxide Solids. Hydrothermal Syntheses and Structures of the Terpyridine Vanadates [V2O4(Terpy)2]3[V10O28], [VO2(Terpy)][V4O10], and [V9O22(Terpy)3]. Inorg. Chem. 2000, 39, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. The automatic searching for chemical bonds in inorganic crystal structures. Acta Cryst. 1985, B41, 244. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.B.; Yang, G.Y. [{(H2O)Ni(enMe)2MoV4MoVI4VIV8(VVO4)O40}2{Ni(enMe)2}][Ni(enMe)2]4∙8H2O: The First Dimer of Polyoxometalates Linked through Coordination Fragment. Chem. Lett. 2002, 31, 1238. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, T.T.; Lin, P.H.; Zhang, X.; Cui, X.B.; Huo, Q.S.; Xu, J.Q. Preparation, structure and characterization of a series of vanadates. CrystEngComm 2017, 19, 265. [Google Scholar] [CrossRef]
- Keene, T.D.; D’Alessandro, D.M.; Krämer, K.W.; Price, J.R.; Price, D.J.; Decurtins, S.; Kepert, C.J. [V16O38(CN)]9−: A Soluble Mixed-Valence Redox-Active Building Block with Strong Antiferromagnetic Coupling. Inorg. Chem. 2012, 51, 9192–9199. [Google Scholar] [CrossRef] [PubMed]
- Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y.; Kaichev, V.V.; Kholdeeva, O.A. Mesoporous niobium-silicates prepared by evaporation-induced self-assembly as catalysts for selective oxidations with aqueous H2O2. J. Catal. 2015, 332, 138. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Evtushok, V.Y.; Maksimov, G.M.; Kholdeeva, O.A. Selective oxidation of pseudocumene and 2-methylnaphthalene with aqueous hydrogen peroxide catalyzed by γ-Keggin divanadium-substituted polyoxotungstate. J. Organomet. Chem. 2015, 793, 210–216. [Google Scholar] [CrossRef]
- Nyman, M.; Burns, P.C. A comprehensive comparison of transition-metal and actinyl polyoxometalates. Chem. Soc. Rev. 2012, 41, 7354–7367. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, G.; Zhao, H. Polyoxometalate as effective catalyst for the deep desulfurization of diesel oil. Catal. Today 2010, 149, 117. [Google Scholar] [CrossRef]
- Gao, H.C.; Yan, Y.; Xu, X.H.; Yu, J.H.; Niu, H.L.; Gao, W.X.; Zhang, W.X.; Jia, M.J. Kinetics and mechanism of thymine degradation by TiO2 photocatalysis. Chin. J. Catal. 2015, 36, 1811–1824. [Google Scholar] [CrossRef]
- Song, X.J.; Yan, Y.; Wang, Y.N.; Hu, D.W.; Xiao, L.N.; Yu, J.H.; Zhang, W.X.; Jia, M.J. Hybrid compounds assembled from copper-triazole complexes and phosphomolybdic acid as advanced catalysts for the oxidation of olefins with oxygen. Dalton Trans. 2017, 46, 16655–16662. [Google Scholar] [CrossRef]
- Ozeki, T.; Yamase, T.; Naruke, H.; Sasaki, Y. X-Ray Structural Characterization of the Protonation Sites in the Dihydrogenhexaniobate Anion. Bull. Chem. Soc. Jpn. 1994, 67, 3249–3253. [Google Scholar] [CrossRef]
- Huang, P.; Qin, C.; Su, Z.M.; Xing, Y.; Wang, X.L.; Shao, K.Z.; Lan, Y.Q.; Wang, E.B. Self-Assembly and Photocatalytic Properties of Polyoxoniobates: {Nb24O72}, {Nb32O96}, and {K12Nb96O288} Clusters. J. Am. Chem. Soc. 2012, 134, 14004–14010. [Google Scholar] [CrossRef]



| Head 1 | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|
| Empirical formula | C52H83Cd4Ge8N12O61.5V12 | C36H78Cd4.5Ge8N13O56V12 | C24H109Cd6Ge6N24O54.5V15 |
| Formula weight | 3501.90 | 3286.91 | 3480.39 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| space group | P-1 | C 2/c | P21/n |
| a (Å) | 14.5034(8) | 17.193(3) | 17.9913(3) |
| b (Å) | 16.5920(9) | 23.511(5) | 23.6117(4) |
| c (Å) | 23.0440(13) | 26.373(5) | 23.9327(4) |
| α (˚) | 71.648(4) | 90 | 90 |
| β (˚) | 84.130(4) | 100.15(3) | 91.7290(13) |
| γ (˚) | 75.454(4) | 90 | 90 |
| Volume (Å3) | 5093.0(5) | 10,494(4) | 10,162.1(3) |
| Z | 2 | 4 | 4 |
| DC (Mg∙m−3) | 2.284 | 2.080 | 2.275 |
| μ (mm−1) | 4.282 | 4.242 | 4.367 |
| F(000) | 3390 | 6324 | 6728 |
| θ for data collection | 1.375–25.032 | 3.025–27.466 | 3.083–29.145 |
| Reflections collected | 28,997 | 45,848 | 54,419 |
| Reflections unique | 17,941 | 11,814 | 23,431 |
| R(int) | 0.1263 | 0.1080 | 0.0437 |
| Completeness to θ | 99.6 | 99.1 | 99.6 |
| parameters | 1360 | 662 | 1207 |
| GOF on F2 | 1.030 | 1.042 | 1.027 |
| R a [I > 2σ(I)] | R1 = 0.0621 | R1 = 0.0822 | R1 = 0.0780 |
| R b (all data) | ωR2 = 0.1660 | ωR2 = 0.2629 | ωR2 = 0.2417 |
| Catalyst | Styrene Conversion a (%) | Product Selectivity b (mol%) | ||
|---|---|---|---|---|
| S | Bza | Others | ||
| GeO2 | 24.8 | 58.6 | 39.8 | 1.7 |
| V2O5 | 71.2 | 67.6 | 28.6 | 3.7 |
| Compound 1 | 50.1 | 62.8 | 34.0 | 3.2 |
| Compound 2 | 96.3 | 71.6 | 16.1 | 12.3 |
| Compound 3 | 81.4 | 63.0 | 34.8 | 2.2 |
| Compound 4 | 84.1 | 55.5 | 39.3 | 5.1 |
| Compound 5 | 41.7 | 67.1 | 32.9 | 0.0 |
| Compound 3 | Styrene Conversion a (%) | Product Selectivity b (mol%) | ||
|---|---|---|---|---|
| S | Bza | Others | ||
| 1st run | 81.4 | 63.0 | 34.8 | 2.2 |
| 2nd run | 54.3 | 59.3 | 37.8 | 2.9 |
| 3rd run | 44.0 | 43.9 | 53.0 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.-Y.; Qi, H.; Zhang, X.; Cui, X.-B. First Organic–Inorganic Hybrid Compounds Formed by Ge-V-O Clusters and Transition Metal Complexes of Aromatic Organic Ligands. Molecules 2022, 27, 4424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144424
Guo H-Y, Qi H, Zhang X, Cui X-B. First Organic–Inorganic Hybrid Compounds Formed by Ge-V-O Clusters and Transition Metal Complexes of Aromatic Organic Ligands. Molecules. 2022; 27(14):4424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144424
Chicago/Turabian StyleGuo, Hai-Yang, Hui Qi, Xiao Zhang, and Xiao-Bing Cui. 2022. "First Organic–Inorganic Hybrid Compounds Formed by Ge-V-O Clusters and Transition Metal Complexes of Aromatic Organic Ligands" Molecules 27, no. 14: 4424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144424