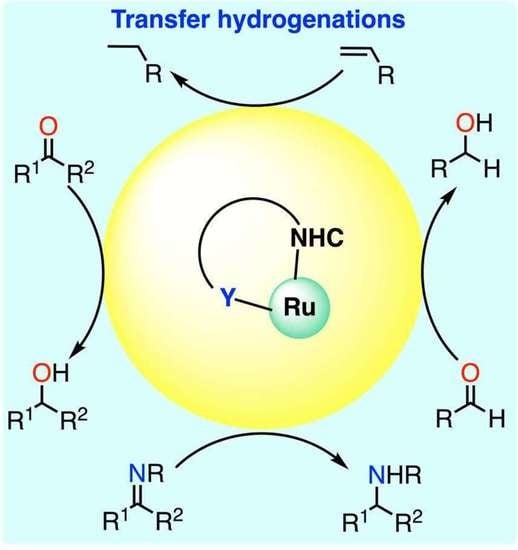
Bidentate Donor-Functionalized N-Heterocyclic Carbenes: Valuable Ligands for Ruthenium-Catalyzed Transfer Hydrogenation
Abstract
:1. Introduction
2. Transfer Hydrogenation of Ketones
2.1. Ketones’ Transfer Hydrogenations with (κ2-CNHC,N)-Complexes
2.2. Ketones’ Transfer Hydrogenations with (κ2-CNHC,P)-Complexes
2.3. Ketones’ Transfer Hydrogenations with (κ2-CNHC,O)-Complexes
3. Asymmetric Transfer Hydrogenation (ATH) of Ketones
3.1. Ketones’ Asymmetric Transfer Hydrogenation with (κ2-CNHC,N)-Complexes
3.2. Ketones’ Asymmetric Transfer Hydrogenation with (κ2-CNHC,O)-Complexes
4. Transfer Hydrogenation of Aldehydes
5. Transfer Hydrogenation of Imines
5.1. Aldimines TH with (κ2-CNHC,N)-Complexes
5.2. Aldimines TH with (κ2-CNHC,O)-Complexes
6. Transfer Hydrogenation of Alkenes
6.1. Alkenes TH with (κ2-C,N)-Complexes
6.2. Alkenes TH with (κ2-C,O)-Complexes
6.3. Alkenes TH with (κ2-C,S)-Complexes
7. Miscellaneous Transfer Hydrogenation
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Zassinovich, G.; Mestroni, G.; Gladiali, S. Asymmetric hydrogen transfer reactions promoted by homogeneous transition metal catalysts. Chem. Rev. 1992, 92, 1051–1069. [Google Scholar] [CrossRef]
- Noyori, R.; Hashiguchi, S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997, 30, 97–102. [Google Scholar] [CrossRef]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2005, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hey, D.A.; Reich, R.M.; Baratta, W.; Kühn, F.E. Current advances on ruthenium(II) N-heterocyclic carbenes in hydrogenation reactions. Coord. Chem. Rev. 2018, 374, 114–132. [Google Scholar] [CrossRef] [Green Version]
- Ritleng, V.; de Vries, J. Ruthenacycles and Iridacycles as Transfer Hydrogenation Catalysts. Molecules 2021, 26, 4076. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Denisova, E.; Eremin, D.B.; Ananikov, V.P. The key role of R–NHC coupling (R = C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species. Chem. Sci. 2020, 11, 6957–6977. [Google Scholar] [CrossRef]
- Normand, A.T.; Cavell, K.J. Donor-Functionalised N-Heterocyclic Carbene Complexes of Group 9 and 10 Metals in Catalysis: Trends and Directions. Eur. J. Inorg. Chem. 2008, 2008, 2781–2800. [Google Scholar] [CrossRef]
- Neshat, A.; Mastrorilli, P.; Mobarakeh, A.M. Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands. Molecules 2021, 27, 95. [Google Scholar] [CrossRef]
- Poyatos, M.; Maisse-François, A.; Bellemin-Laponnaz, S.; Peris, E.; Gade, L.H. Synthesis and structural chemistry of arene-ruthenium half-sandwich complexes bearing an oxazolinyl–carbene ligand. J. Organomet. Chem. 2006, 691, 2713–2720. [Google Scholar] [CrossRef]
- Gnanamgari, D.; Sauer, E.L.O.; Schley, N.; Butler, C.; Incarvito, C.D.; Crabtree, R.H. Iridium and Ruthenium Complexes with Chelating N-Heterocyclic Carbenes: Efficient Catalysts for Transfer Hydrogenation, β-Alkylation of Alcohols, and N-Alkylation of Amines. Organometallics 2008, 28, 321–325. [Google Scholar] [CrossRef]
- Wylie, W.N.; Lough, A.J.; Morris, R.H. Transmetalation of a Primary Amino-Functionalized N-Heterocyclic Carbene Ligand from an Axially Chiral Square-Planar Nickel(II) Complex to a Ruthenium(II) Precatalyst for the Transfer Hydrogenation of Ketones. Organometallics 2009, 28, 6755–6761. [Google Scholar] [CrossRef]
- Ohara, H.; Wylie, W.N.; Lough, A.J.; Morris, R.H. Effect of chelating ring size in catalytic ketone hydrogenation: Facile synthesis of ruthenium(ii) precatalysts containing an N-heterocyclic carbene with a primary amine donor for ketone hydrogenation and a DFT study of mechanisms. Dalton Trans. 2012, 41, 8797–8808. [Google Scholar] [CrossRef] [PubMed]
- Cross, W.B.; Daly, C.G.; Boutadla, Y.; Singh, K. Variable coordination of amine functionalised N-heterocyclic carbene ligands to Ru, Rh and Ir: C–H and N–H activation and catalytic transfer hydrogenation. Dalton Trans. 2011, 40, 9722–9730. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.E.; Puerta, M.C.; Valerga, P. Half-Sandwich Ruthenium(II) Picolyl-NHC Complexes: Synthesis, Characterization, and Catalytic Activity in Transfer Hydrogenation Reactions. Organometallics 2011, 30, 5793–5802. [Google Scholar] [CrossRef]
- Ning, J.; Shang, Z.; Xu, X. How Does the Hemilabile Group in Ruthenium-Cp* Picolyl-NHC Complexes Affect the Mechanism of Transfer Hydrogenation Reaction? A DFT Study. Catal. Lett. 2015, 145, 1331–1343. [Google Scholar] [CrossRef]
- Fernández, F.E.; Puerta, M.C.; Valerga, P. Ruthenium(II) Picolyl-NHC Complexes: Synthesis, Characterization, and Catalytic Activity in Amine N-alkylation and Transfer Hydrogenation Reactions. Organometallics 2012, 31, 6868–6879. [Google Scholar] [CrossRef]
- Piyasaengthong, A.; Williams, L.J.; Yufit, D.S.; Walton, J.W. Novel ruthenium complexes bearing bipyridine-based and N-heterocyclic carbene-supported pyridine (NCN) ligands: The influence of ligands for catalytic transfer hydrogenation of ketones. Dalton Trans. 2022, 51, 340–351. [Google Scholar] [CrossRef]
- Pámies, O.; Bäckvall, J.-E. Studies on the Mechanism of Metal-Catalyzed Hydrogen Transfer from Alcohols to Ketones. Chem. Eur. J. 2001, 7, 5052–5058. [Google Scholar] [CrossRef]
- Albrecht, M.; Miecznikowski, J.; Samuel, A.; Faller, J.; Crabtree, R.H. Chelated Iridium(III) Bis-carbene Complexes as Air-Stable Catalysts for Transfer Hydrogenation. Organometallics 2002, 21, 3596–3604. [Google Scholar] [CrossRef]
- Guo, X.-Q.; Wang, Y.-N.; Wang, D.; Cai, L.-H.; Chen, Z.-X.; Hou, X.-F. Palladium, iridium and ruthenium complexes with acyclic imino-N-heterocyclic carbenes and their application in aqua-phase Suzuki–Miyaura cross-coupling reaction and transfer hydrogenation. Dalton Trans. 2012, 41, 14557–14567. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rebollo, M.; Canseco-Gonzalez, D.; Hollering, M.; Mueller-Bunz, H.; Albrecht, M. Synthesis and catalytic alcohol oxidation and ketone transfer hydrogenation activity of donor-functionalized mesoionic triazolylidene ruthenium(ii) complexes. Dalton Trans. 2013, 43, 4462–4473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolje, A.; Hohloch, S.; van der Meer, M.; Košmrlj, J.; Sarkar, B. RuII, OsII, and IrIIIComplexes with Chelating Pyridyl-Mesoionic Carbene Ligands: Structural Characterization and Applications in Transfer Hydrogenation Catalysis. Chem.-A Eur. J. 2015, 21, 6756–6764. [Google Scholar] [CrossRef]
- Bolje, A.; Hohloch, S.; Košmrlj, J.; Sarkar, B. RuII, IrIII and OsII mesoionic carbene complexes: Efficient catalysts for transfer hydrogenation of selected functionalities. Dalton Trans. 2016, 45, 15983–15993. [Google Scholar] [CrossRef] [Green Version]
- Olguín, J.; Paz-Sandoval, M. Synthesis and transfer hydrogenation catalysis of chelating triazolylidene ruthenium(II) complexes: Effect of the pendant arm, p-cymene, acetonitrile and butadienesulfonyl co-ligands. J. Organomet. Chem. 2017, 848, 309–317. [Google Scholar] [CrossRef]
- Sabater, S.; Müller-Bunz, H.; Albrecht, M. Carboxylate-Functionalized Mesoionic Carbene Precursors: Decarboxylation, Ruthenium Bonding, and Catalytic Activity in Hydrogen Transfer Reactions. Organometallics 2016, 35, 2256–2266. [Google Scholar] [CrossRef]
- Olguín, J.; Díaz-Fernández, M.; de la Cruz-Cruz, J.I.; Paz-Sandoval, M.A. Mixed heteropentadienyl and N-heterocyclic carbene ruthenium(II) complexes: Synthesis and transfer hydrogenation catalysis. J. Organomet. Chem. 2016, 824, 33–41. [Google Scholar] [CrossRef]
- Smith, I.G.; Zgrabik, J.C.; Gutauskas, A.C.; Gray, D.L.; Domski, G.J. Synthesis, characterization, and catalytic behavior of mono- and bimetallic ruthenium(II) and iridium(III) complexes supported by pyridine-functionalized N -heterocyclic carbene ligands. Inorg. Chem. Commun. 2017, 81, 27–32. [Google Scholar] [CrossRef]
- Viji, M.; Tyagi, N.; Naithani, N.; Ramaiah, D. Aryl appended neutral and cationic half-sandwich ruthenium(ii)–NHC complexes: Synthesis, characterisation and catalytic applications. New J. Chem. 2017, 41, 12736–12745. [Google Scholar] [CrossRef]
- Castañón, E.B.; Kaposi, M.; Reich, R.M.; Kühn, F.E. Water-soluble transition metal complexes of ruthenium(ii), osmium(ii), rhodium(iii) and iridium(iii) with chelating N-heterocyclic carbene ligands in hydrogenation and transfer hydrogenation catalysis. Dalton Trans. 2018, 47, 2318–2329. [Google Scholar] [CrossRef]
- Azua, A.; Finn, M.; Yi, H.; Dantas, A.B.; Voutchkova-Kostal, A. Transfer Hydrogenation from Glycerol: Activity and Recyclability of Iridium and Ruthenium Sulfonate-Functionalized N-Heterocyclic Carbene Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 3963–3972. [Google Scholar] [CrossRef]
- Nair, A.G.; McBurney, R.T.; Walker, D.B.; Page, M.J.; Gatus, M.R.D.; Bhadbhade, M.; Messerle, B.A. Ruthenium(ii) complexes of hemilabile pincer ligands: Synthesis and catalysing the transfer hydrogenation of ketones. Dalton Trans. 2016, 45, 14335–14342. [Google Scholar] [CrossRef] [PubMed]
- Hollering, M.; Albrecht, M.; Kühn, F.E. Bonding and Catalytic Application of Ruthenium N-Heterocyclic Carbene Complexes Featuring Triazole, Triazolylidene, and Imidazolylidene Ligands. Organometallics 2016, 35, 2980–2986. [Google Scholar] [CrossRef]
- Illam, P.M.; Donthireddy, S.N.R.; Chakrabartty, S.; Rit, A. Heteroditopic Ru(II)– and Ir(III)–NHC Complexes with Pendant 1,2,3-Triazole/Triazolylidene Groups: Stereoelectronic Impact on Transfer Hydrogenation of Unsaturated Compounds. Organometallics 2019, 38, 2610–2623. [Google Scholar] [CrossRef]
- Sinha, A.; Daw, P.; Rahaman, S.W.; Saha, B.; Bera, J.K. A RuII–N-heterocyclic carbene (NHC) complex from metal–metal singly bonded diruthenium(I) precursor: Synthesis, structure and catalytic evaluation. J. Organomet. Chem. 2011, 696, 1248–1257. [Google Scholar] [CrossRef]
- Yoshimura, M.; Kamisue, R.; Sakaguchi, S. Synthesis of Ru(II) complexes containing N-heterocyclic carbenes functionalized with secondary donor groups: Catalytic activity toward enantioselective transfer hydrogenation. J. Organomet. Chem. 2013, 740, 26–32. [Google Scholar] [CrossRef]
- Sortais, J.-B.; Ritleng, V.; Voelklin, A.; Holuigue, A.; Smail, H.; Barloy, L.; Sirlin, C.; Verzijl, G.K.M.; Boogers, J.A.F.; de Vries, A.H.M.; et al. Cycloruthenated Primary and Secondary Amines as Efficient Catalyst Precursors for Asymmetric Transfer Hydrogenation. Org. Lett. 2005, 7, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Jerphagnon, T.; Haak, R.; Berthiol, F.; Gayet, A.J.A.; Ritleng, V.; Holuigue, A.; Pannetier, N.; Pfeffer, M.; Voelklin, A.; Lefort, L.; et al. Ruthenacycles and Iridacycles as Catalysts for Asymmetric Transfer Hydrogenation and Racemisation. Top. Catal. 2010, 53, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Kilic, A.; Kayan, C.; Aydemir, M.; Durap, F.; Durgun, M.; Baysal, A.; Tas, E.; Gümgüm, B. Synthesis of new boron complexes: Application to transfer hydrogenation of acetophenone derivatives. Appl. Organomet. Chem. 2011, 25, 390–394. [Google Scholar] [CrossRef]
- Kilic, A.; Aydemir, M.; Durgun, M.; Meriç, N.; Ocak, Y.S.; Keles, A.; Temel, H. Fluorine/phenyl chelated boron complexes: Synthesis, fluorescence properties and catalyst for transfer hydrogenation of aromatic ketones. J. Fluor. Chem. 2014, 162, 9–16. [Google Scholar] [CrossRef]
- Leigh, V.; Carleton, D.J.; Olguin, J.; Mueller-Bunz, H.; Wright, L.J.; Albrecht, M. Solvent-Dependent Switch of Ligand Donor Ability and Catalytic Activity of Ruthenium(II) Complexes Containing Pyridinylidene Amide (PYA) N-Heterocyclic Carbene Hybrid Ligands. Inorg. Chem. 2014, 53, 8054–8060. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, K.F.; Segarra, C.; Shao, L.-X.; Suen, R.; Müller-Bunz, H.; Albrecht, M. Adaptive N-Mesoionic Ligands Anchored to a Triazolylidene for Ruthenium-Mediated (De)Hydrogenation Catalysis. Organometallics 2015, 34, 4076–4084. [Google Scholar] [CrossRef]
- Navarro, M.; Segarra, C.; Pfister, T.; Albrecht, M. Structural, Electronic, and Catalytic Modulation of Chelating Pyridylideneamide Ruthenium(II) Complexes. Organometallics 2020, 39, 2383–2391. [Google Scholar] [CrossRef]
- Malan, F.P.; Singleton, E.; van Rooyen, P.H.; Albrecht, M.; Landman, M. Synthesis, Stability, and (De)hydrogenation Catalysis by Normal and Abnormal Alkene- and Picolyl-Tethered NHC Ruthenium Complexes. Organometallics 2019, 38, 2624–2635. [Google Scholar] [CrossRef]
- Albrecht, M. C4-bound imidazolylidenes: From curiosities to high-impact carbene ligands. Chem. Commun. 2008, 3601–3610. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, R.H. Abnormal, mesoionic and remote N-heterocyclic carbene complexes. Coord. Chem. Rev. 2013, 257, 755–766. [Google Scholar] [CrossRef]
- Juzgado, A.; Lorenzo-García, M.M.; Barrejón, M.; Rodríguez, A.M.; Rodríguez-López, J.; Merino, S.; Tejeda, J. Chelation assistance as a tool for the selective preparation of an imidazole-based mesoionic palladium carbene complex. Chem. Commun. 2014, 50, 15313–15315. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, J.-F.; Yang, H.-L.; Xu, H.-J.; Li, Y.-Z.; Chen, X.-T.; Xue, Z.-L. Syntheses, Structures, and Catalytic Properties of Ruthenium(II) Nitrosyl Complexes with Pyridine-Functionalized N-Heterocyclic Carbenes. Organometallics 2009, 28, 819–823. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, H.-J.; Sun, J.-F.; Li, Y.-Z.; Chen, X.-T.; Xue, Z.-L. Synthesis, structures and catalytic activities of ruthenium(ii) carbonyl chloride complexes containing pyridine-functionalised N-heterocyclic carbenes. Dalton Trans. 2009, 7132–7140. [Google Scholar] [CrossRef]
- Li, X.-W.; Wang, G.-F.; Chen, F.; Li, Y.-Z.; Chen, X.-T.; Xue, Z.-L. Ruthenium(II) carbonyl chloride complexes containing pyridine-functionalised bidentate N-heterocyclic carbenes: Synthesis, structures, and impact of the carbene ligands on catalytic activities. Inorg. Chim. Acta 2011, 378, 280–287. [Google Scholar] [CrossRef]
- Chen, C.; Lu, C.; Zheng, Q.; Ni, S.; Zhang, M.; Chen, W. Synthesis and structures of ruthenium–NHC complexes and their catalysis in hydrogen transfer reaction. Beilstein J. Org. Chem. 2015, 11, 1786–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Soto, V.; Grotjahn, D.B.; Cooksy, A.L.; Golen, J.A.; Moore, C.E.; Rheingold, A.L. A Labile and Catalytically Active Imidazol-2-yl Fragment System. Angew. Chem. Int. Ed. 2010, 50, 631–635. [Google Scholar] [CrossRef]
- Kuwata, S.; Ikariya, T. Metal-ligand bifunctional reactivity and catalysis of protic N-heterocyclic carbene and pyra-zole complexes featuring β-NH units. Chem. Commun. 2014, 50, 14290–14300. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.E.; Pecak, W.H.; Hohenboken, S.A.; Alvarado, S.R.; Swenson, D.C.; Domski, G.J. Ruthenium(II) supported by phosphine-functionalized N-heterocyclic carbene ligands as catalysts for the transfer hydrogenation of ketones. Inorg. Chem. Commun. 2013, 37, 138–143. [Google Scholar] [CrossRef]
- Witt, J.; Pöthig, A.; Kühn, F.E.; Baratta, W. Abnormal N-Heterocyclic Carbene-Phosphine Ruthenium(II) Complexes as Active Catalysts for Transfer Hydrogenation. Organometallics 2013, 32, 4042–4045. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ito, H.; Noyori, R. The Metal−Ligand Bifunctional Catalysis: A Theoretical Study on the Ruthenium(II)-Catalyzed Hydrogen Transfer between Alcohols and Carbonyl Compounds. J. Am. Chem. Soc. 2000, 122, 1466–1478. [Google Scholar] [CrossRef]
- Bitzer, M.J.; Pöthig, A.; Jandl, C.; Kühn, F.E.; Baratta, W. Ru–Ag and Ru–Au dicarbene complexes from an abnormal carbene ruthenium system. Dalton Trans. 2015, 44, 11686–11689. [Google Scholar] [CrossRef] [Green Version]
- Pardatscher, L.; Bitzer, M.J.; Jandl, C.; Kück, J.W.; Reich, R.M.; Kühn, F.E.; Baratta, W. Cationic abnormal N-heterocyclic carbene ruthenium complexes as suitable precursors for the synthesis of heterobimetallic compounds. Dalton Trans. 2018, 48, 79–89. [Google Scholar] [CrossRef]
- Pardatscher, L.; Hofmann, B.J.; Fischer, P.J.; Hölzl, S.M.; Reich, R.M.; Kühn, F.E.; Baratta, W. Highly Efficient Abnormal NHC Ruthenium Catalyst for Oppenauer-Type Oxidation and Transfer Hydrogenation Reactions. ACS Catal. 2019, 9, 11302–11306. [Google Scholar] [CrossRef]
- Chelucci, G.; Baldino, S.; Baratta, W. Ruthenium and osmium complexes containing 2-(aminomethyl)pyridine (Ampy)-based ligands in catalysis. Coord. Chem. Rev. 2015, 300, 29–85. [Google Scholar] [CrossRef]
- Baratta, W.; Schütz, J.; Herdtweck, E.; Herrmann, W.A.; Rigo, P. Fast transfer hydrogenation using a highly active orthometalated heterocyclic carbene ruthenium catalyst. J. Organomet. Chem. 2005, 690, 5570–5575. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Falomir, E.; Crabtree, R.H.; Peris, E. New Ruthenium(II) CNC-Pincer Bis(carbene) Complexes: Synthesis and Catalytic Activity. Organometallics 2003, 22, 1110–1114. [Google Scholar] [CrossRef]
- Hollering, M.; Weiss, D.T.; Bitzer, M.J.; Jandl, C.; Kühn, F.E. Controlling Coordination Geometries: Ru–Carbene Complexes with Tetra-NHC Ligands. Inorg. Chem. 2016, 55, 6010–6017. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Gandolfi, C.; Albrecht, M. Transfer Hydrogenation of Ketones and Activated Olefins Using Chelating NHC Ruthenium Complexes. Eur. J. Inorg. Chem. 2011, 2011, 2863–2868. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, C.; Heckenroth, M.; Neels, A.; Laurenczy, G.; Albrecht, M. Chelating NHC Ruthenium(II) Complexes as Robust Homogeneous Hydrogenation Catalysts. Organometallics 2009, 28, 5112–5121. [Google Scholar] [CrossRef] [Green Version]
- DePasquale, J.; White, N.J.; Ennis, E.J.; Zeller, M.; Foley, J.P.; Papish, E.T. Synthesis of chiral N-heterocyclic carbene (NHC) ligand precursors and formation of ruthenium(II) complexes for transfer hydrogenation catalysts. Polyhedron 2012, 58, 162–170. [Google Scholar] [CrossRef]
- Mangalum, A.; McMillen, C.D.; Tennyson, A. Synthesis, coordination chemistry and reactivity of transition metal complexes supported by a chelating benzimidazolylidene carboxylate ligand. Inorg. Chim. Acta 2015, 426, 29–38. [Google Scholar] [CrossRef]
- Akkoç, M.; Öz, E.; Demirel, S.; Dorcet, V.; Roisnel, T.; Bayri, A.; Bruneau, C.; Altin, S.; Yaşar, S.; Özdemir, I. Investigation of potential hybrid capacitor property of chelated N-Heterocyclic carbene Ruthenium(II) complex. J. Organomet. Chem. 2018, 866, 214–222. [Google Scholar] [CrossRef]
- Pretorius, R.; Mazloomi, Z.; Albrecht, M. Synthesis, hemilability, and catalytic transfer hydrogenation activity of iridium(III) and ruthenium(II) complexes containing oxygen-functionalised triazolylidene ligands. J. Organomet. Chem. 2017, 845, 196–205. [Google Scholar] [CrossRef]
- Wan, K.Y.; Lough, A.J.; Morris, R.H. Transition Metal Complexes of an (S,S)-1,2-Diphenylethylamine-Functionalized N-Heterocyclic Carbene: A New Member of the Asymmetric NHC Ligand Family. Organometallics 2016, 35, 1604–1612. [Google Scholar] [CrossRef] [Green Version]
- Horn, S.; Albrecht, M. Transfer hydrogenation of unfunctionalised alkenes using N-heterocyclic carbene ruthenium catalyst precursors. Chem. Commun. 2011, 47, 8802–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohloch, S.; Suntrup, L.; Sarkar, B. Arene–Ruthenium(II) and −Iridium(III) Complexes with “Click”-Based Pyridyl-triazoles, Bis-triazoles, and Chelating Abnormal Carbenes: Applications in Catalytic Transfer Hydrogenation of Nitrobenzene. Organometallics 2013, 32, 7376–7385. [Google Scholar] [CrossRef]















































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritleng, V.; Michon, C. Bidentate Donor-Functionalized N-Heterocyclic Carbenes: Valuable Ligands for Ruthenium-Catalyzed Transfer Hydrogenation. Molecules 2022, 27, 4703. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27154703
Ritleng V, Michon C. Bidentate Donor-Functionalized N-Heterocyclic Carbenes: Valuable Ligands for Ruthenium-Catalyzed Transfer Hydrogenation. Molecules. 2022; 27(15):4703. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27154703
Chicago/Turabian StyleRitleng, Vincent, and Christophe Michon. 2022. "Bidentate Donor-Functionalized N-Heterocyclic Carbenes: Valuable Ligands for Ruthenium-Catalyzed Transfer Hydrogenation" Molecules 27, no. 15: 4703. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27154703