Data from Multiple Portable XRF Units and Their Significance for Ancient Glass Studies
Abstract
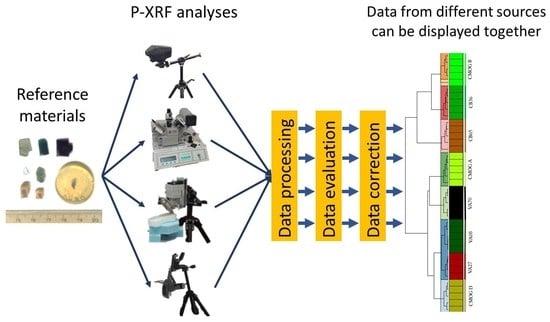
:1. Introduction
2. Materials and Methods
2.1. Reference Glasses
2.2. XRF Units and Settings
- XGLab ELIO (hereafter ELIO). The commercial unit is produced by XGLab S.R.L. (Milan, Italy);
- Unisantis XMF-104 (hereafter Unisantis). The commercial unit is equipped with polycapillary optics and produced by Unistantis (Geneva, Switzerland). Regarding the size and configuration of the instrument, this is the only one with limited sample size analysis due to the dimensions of the sample chamber and can therefore be included in the category of transportable X-ray spectrometers [52];
- Frankie (hereafter Frankie). An ad hoc unit with policapillary optics, developed by the Italian National Institute of Nuclear Physics-National Laboratories of Frascati ((INFN-LNF) Italy);
- NITON XL3T-900 GOLDD (hereafter NITON). The commercial unit is by Thermo Fisher Scientific (Waltham, MA, USA). This instrument acquires a series of spectra in different energy ranges to enhance the signal in specific parts of the spectrum.
2.3. Acquisition and Processing
3. Results and Discussion
3.1. Estimation of Limits of Quantification
3.2. Precision of the Measurements
3.3. Accuracy with the Fundamental Parameters Approach
3.4. Ratio Coefficients Correction
3.5. Linear Regression Correction
3.6. Multivariate Approach to the Reproducibility Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesareo, R.; Frazzoli, F.V.; Mancini, C.; Sciuti, S.; Marabelli, A.M.; Mora, P.; Rotondi, P.; Urbani, G. Non-Destructive Analysis of Chemical Elements in Paintings and Enamels. Archaeometry 1972, 14, 65–78. [Google Scholar] [CrossRef]
- Hall, E.T.; Schweizer, F.; Toller, P.A. X-ray fluorescence analysis of museum objects: A new instrument. Archaeometry 1973, 15, 53–78. [Google Scholar] [CrossRef]
- Liritzis, I.; Zacharias, N. Portable XRF of Archaeological Artifacts: Current Research, Potentials and Limitations. In X-Ray Fluorescence Spectrometry (XRF) in Geoarchaeology; Shackley, M., Ed.; Springer: New York, NY, USA, 2011; pp. 109–142. [Google Scholar] [CrossRef]
- Frahm, E.; Doonan, R.C. The technological versus methodological revolution of portable XRF in archaeology. J. Archaeol. Sci. 2013, 40, 1425–1434. [Google Scholar] [CrossRef]
- Shugar, A.N. Portable X-ray Fluorescence and Archaeology: Limitations of the Instrument and Suggested Methods to Achieve Desired Results. In Archaeological Chemistry VIII; Armithage, R.A., Burton, J.H., Eds.; American Chemical Society: Washington, DC, USA, 2013; pp. 173–193. [Google Scholar] [CrossRef]
- Scott, R.; Shortland, A.; Degryse, P.; Power, M.; Domoney, K.; Boyen, S.; Braekmans, D. In situ analysis of ancient glass: 17th century painted glass from Christ Church Cathedral, Oxford and Roman glass vessels. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2012, 53, 65–73. [Google Scholar]
- Janssens, K. X-ray Based Methods of Analysis. In Modern Methods for Analysing Archaeological and Historical Glass; Janssens, K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 79–126. [Google Scholar] [CrossRef]
- Micheletti, F.; Orsilli, J.; Melada, J.; Gargano, M.; Ludwig, N.; Bonizzoni, L. The role of IRT in the archaeometric study of ancient glass through XRF and FORS. Microchem. J. 2019, 153, 104388. [Google Scholar] [CrossRef]
- Abe, Y.; Shikaku, R.; Murakushi, M.; Fukushima, M.; Nakai, I. Did ancient glassware travel the Silk Road? X-ray fluorescence analysis of a Sasanian glass vessel from Okinoshima Island, Japan. J. Archaeol. Sci. Rep. 2021, 40, 103195. [Google Scholar] [CrossRef]
- Longoni, A.; Fiorini, C. Detectors and Signal Processing. In Handbook of Practical X-ray Fluorescence Analysis; Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., Wolff, H., Eds.; Springer Science & Business Media: Berlin, Germany, 2007; pp. 203–261. [Google Scholar] [CrossRef]
- Tantrakarn, K.; Kato, N.; Hokura, A.; Nakai, I.; Fujii, Y.; Gluščević, S. Archaeological analysis of Roman glass excavated from Zadar, Croatia, by a newly developed portable XRF spectrometer for glass. X-Ray Spectrom. 2009, 38, 121–127. [Google Scholar] [CrossRef]
- Migliori, A.; Bonanni, P.; Carraresi, L.; Grassi, N.; Mandò, P.A. A novel portable XRF spectrometer with range of detection extended to low-Z elements. X-Ray Spectrom. 2011, 40, 107–112. [Google Scholar] [CrossRef]
- Adams, C.; Brand, C.; Dentith, M.; Fiorentini, M.; Caruso, S.; Mehta, M. The use of pXRF for light element geochemical analysis: A review of hardware design limitations and an empirical investigation of air, vacuum, helium flush and detector window technologies. Geochem. Explor. Environ. Anal. 2020, 20, 366–380. [Google Scholar] [CrossRef]
- Conrey, R.M.; Goodman-Elgar, M.; Bettencourt, N.; Seyfarth, A.; Van Hoose, A.; Wolff, J.A. Calibration of a portable X-ray fluorescence spectrometer in the analysis of archaeological samples using influence coefficients. Geochem. Explor. Environ. Anal. 2014, 14, 291–301. [Google Scholar] [CrossRef]
- de Winter, N.J.; Sinnesael, M.; Makarona, C.; Vansteenberge, S.; Claeys, P. Trace element analyses of carbonates using portable and micro-X-ray fluorescence: Performance and optimization of measurement parameters and strategies. J. Anal. At. Spectrom. 2017, 32, 1211–1223. [Google Scholar] [CrossRef]
- Bezur, A.; Fong Lee, L.; Loubser, M.; Trentelman, K. Handheld XRF in Cultural Heritage: A Practical Workbook for Conservators; Getty Conservation Institute: Los-Angeles, CA, USA, 2020. [Google Scholar]
- Knight, R.D.; Kjarsgaard, B.A.; Russell, H.A. An analytical protocol for determining the elemental chemistry of Quaternary sediments using a portable X-ray fluorescence spectrometer. Appl. Geochem. 2021, 131, 105026. [Google Scholar] [CrossRef]
- Frahm, E. Characterizing obsidian sources with portable XRF: Accuracy, reproducibility, and field relationships in a case study from Armenia. J. Archaeol. Sci. 2014, 49, 105–125. [Google Scholar] [CrossRef]
- Duckworth, C.N. Latest advancements in the application of analytical science to ancient and historical glass production. UISPP J. 2019, 2, 99–110. [Google Scholar]
- Odelli, E.; Rousaki, A.; Raneri, S.; Vandenabeele, P. Advantages and pitfalls of the use of mobile Raman and XRF systems applied on cultural heritage objects in Tuscany (Italy). Eur. Phys. J. Plus 2021, 136, 449. [Google Scholar] [CrossRef]
- Legrand, S.; Van der Snickt, G.; Cagno, S.; Caen, J.; Janssens, K. MA-XRF imaging as a tool to characterize the 16th century heraldic stained-glass panels in Ghent Saint Bavo Cathedral. J. Cult. Herit. 2019, 40, 163–168. [Google Scholar] [CrossRef]
- Cagno, S.; Van der Snickt, G.; Legrand, S.; Caen, J.; Patin, M.; Meulebroeck, W.; Dirkx, Y.; Hillen, M.; Steenackers, G.; Rousaki, A.; et al. Comparison of four mobile, non-invasive diagnostic techniques for differentiating glass types in historical leaded windows: MA-XRF, UV–Vis–NIR, Raman spectroscopy and IRT. X-Ray Spectrom. 2020, 50, 293–309. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Standard 5725-1. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:ed-1:v1:en (accessed on 22 July 2022).
- Hein, A.; Dobosz, A.; Day, P.M.; Kilikoglou, V. Portable ED-XRF as a tool for optimizing sampling strategy: The case study of a Hellenistic amphora assemblage from Paphos (Cyprus). J. Archaeol. Sci. 2021, 133, 105436. [Google Scholar] [CrossRef]
- Costa, M.; Barrulas, P.; Dias, L.; Lopes, M.D.C.; Barreira, J.; Clist, B.; Karklins, K.; Jesus, M.D.P.D.; Domingos, S.D.S.; Vandenabeele, P.; et al. Multi-analytical approach to the study of the European glass beads found in the tombs of Kulumbimbi (Mbanza Kongo, Angola). Microchem. J. 2019, 149, 103990. [Google Scholar] [CrossRef]
- Hodgkinson, A.K.; Röhrs, S.; Müller, K.; Reiche, I. The use of Cobalt in 18th Dynasty Blue Glass from Amarna: The results from an on-site analysis using portable XRF technology. STAR Sci. Technol. Archaeol. Res. 2019, 5, 36–52. [Google Scholar] [CrossRef]
- Demirsar Arli, B.; Simsek Franci, G.; Kaya, S.; Arli, H.; Colomban, P. Portable X-ray Fluorescence (p-XRF) Uncertainty Estimation for Glazed Ceramic Analysis: Case of Iznik Tiles. Heritage 2020, 3, 1302–1329. [Google Scholar] [CrossRef]
- Spencer, H.M.; Murdoch, K.R.; Buckman, J.; Forster, A.M.; Kennedy, C.J. Compositional Analysis by p-XRF and SEM-EDX of Medieval Window Glass from Elgin Cathedral, Northern Scotland. Archaeometry 2018, 60, 1018–1035. [Google Scholar] [CrossRef]
- Bruni, Y.; Hatert, F.; George, P.; Strivay, D. The Reliquary Bust of Saint Lambert from the Liège Cathedral, Belgium: Gemstones and Glass Beads Analysis by pXRF and Raman Spectroscopy. Archaeometry 2019, 62, 297–313. [Google Scholar] [CrossRef]
- Abe, Y.; Shikaku, R.; Yamamoto, M.; Yagi, N.; Nakai, I. Ancient glassware travelled the Silk Road: Nondestructive X-ray fluorescence analysis of a fragment of a facet-cut glass vessel collected at Kamigamo Shrine in Kyoto, Japan. J. Archaeol. Sci. Rep. 2018, 20, 362–368. [Google Scholar] [CrossRef]
- Liritzis, I.; Zacharias, N.; Papageorgiou, I.; Tsarucha, A. Characterisation and analyses of museum objects using pXRF: An application from the Delphi Museum, Greece. Studia Antiq. Archaeol. 2018, 24, 31–50. [Google Scholar]
- Koleini, F.; Pikirayi, I.; Colomban, P. Revisiting Baranda: A multi-analytical approach in classifying sixteenth/seventeenth-century glass beads from northern Zimbabwe. Antiquity 2017, 91, 751–764. [Google Scholar] [CrossRef]
- Fischer, C.; Hsieh, E. Export Chinese blue-and-white porcelain: Compositional analysis and sourcing using non-invasive portable XRF and reflectance spectroscopy. J. Archaeol. Sci. 2017, 80, 14–26. [Google Scholar] [CrossRef]
- de Boer, D. Fundamental parameters for X-ray fluorescence analysis. Spectrochim. Acta Part B At. Spectrosc. 1989, 44, 1171–1190. [Google Scholar] [CrossRef]
- Sokaras, D.; Karydas, A.G.; Oikonomou, A.; Zacharias, N.; Beltsios, K.; Kantarelou, V. Combined elemental analysis of ancient glass beads by means of ion beam, portable XRF, and EPMA techniques. Anal. Bioanal. Chem. 2009, 395, 2199–2209. [Google Scholar] [CrossRef]
- Heginbotham, A.; Bezur, A.; Bouchard, M.; Davis, J.; Eremin, K.; Frantz, J.; Glinsman, L.; Hayek, L.A.; Hook, D.; Kantarelou, V.; et al. An evaluation of in-ter-laboratory reproducibility for quantitative XRF of historic copper alloys. In Proceedings of the METAL 2010, the Interim Meeting of the ICOM-CC Metal Working Group, Charlestown, NC, USA, 11–15 October 2010. [Google Scholar]
- Brand, N.W.; Brand, C.J. Performance comparison of portable XRF instruments. Geochem. Explor. Environ. Anal. 2014, 14, 125–138. [Google Scholar] [CrossRef]
- Sarala, P. Comparison of different portable XRF methods for determining till geochemistry. Geochem. Explor. Environ. Anal. 2016, 16, 181–192. [Google Scholar] [CrossRef]
- Declercq, Y.; Delbecque, N.; De Grave, J.; De Smedt, P.; Finke, P.; Mouazen, A.M.; Nawar, S.; Vandenberghe, D.; Van Meirvenne, M.; Verdoodt, A. A Comprehensive Study of Three Different Portable XRF Scanners to Assess the Soil Geochemistry of An Extensive Sample Dataset. Remote Sens. 2019, 11, 2490. [Google Scholar] [CrossRef]
- Heginbotham, A.; Solé, V.A. CHARMed PyMca, Part I: A Protocol for Improved Inter-laboratory Reproducibility in the Quantitative ED-XRF Analysis of Copper Alloys. Archaeometry 2017, 59, 714–730. [Google Scholar] [CrossRef]
- Heginbotham, A.; Bourgarit, D.; Day, J.; Dorscheid, J.; Godla, J.; Lee, L.; Pappot, A.; Robcis, D. CHARMed PyMca, Part II: An evaluation of interlaboratory reproducibility for ED-XRF analysis of copper alloys. Archaeometry 2019, 61, 1333–1352. [Google Scholar] [CrossRef]
- Henderson, J. Ways to Flux Silica: Ashes and Minerals. In Ancient Glass: An Interdisciplinary Exploration; Henderson, J., Ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 22–55. [Google Scholar] [CrossRef]
- Brill, R.H. A chemical-analytical round-robin on four synthetic ancient glasses. In IX Congreès International du Verre: Communications Artistiques et Historique (Versailles, 1971); The Corning Museum of Glass: Corning, NY, USA, 1972; pp. 93–110. [Google Scholar]
- Reade, W.; Freestone, I.C.; Bourke, S. Innovation and continuity in Bronze and Iron Age glass from Pella in Jordan. In Proceedings of the 17th Congress of the Association Internationale Pour l’Histoire du Verre, Antwerp, Belgium, 4–8 September 2006. [Google Scholar]
- Walton, M.; Eremin, K.; Shortland, A.; Degryse, P.; Kirk, S. Analysis of Late Bronze Age glass axes from Nippur—A new cobalt colourant. Archaeometry 2012, 54, 835–852. [Google Scholar] [CrossRef]
- Brill, R.H. Chemical Analyses of Early Glasses; Corning Museum of Glass: Corning, NY, USA, 1999; Volume 2, pp. 529–553. [Google Scholar]
- Adlington, L.W. The Corning Archaeological Reference Glasses: New Values for “Old” Compositions. Pap. Inst. Archaeol. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Mirti, P.; Pace, M.; Ponzi, M.M.N.; Aceto, M. ICP–MS Analysis of Glass Fragments of Parthian and Sasanian Epoch from Seleucia and Veh Ardašīr (Central Iraq). Archaeometry 2007, 50, 429–450. [Google Scholar] [CrossRef]
- Mirti, P.; Pace, M.; Malandrino, M.; Ponzi, M.N. Sasanian glass from Veh Ardašīr: New evidences by ICP-MS analysis. J. Archaeol. Sci. 2009, 36, 1061–1069. [Google Scholar] [CrossRef]
- Mirti, P.; Davit, P.; Gulmini, M.; Sagui, L. Glass Fragments from the Crypta Balbi in Rome: The Composition of Eighth-century Fragments. Archaeometry 2001, 43, 491–502. [Google Scholar] [CrossRef]
- Mirti, P.; Lepora, A.; Saguì, L. Scientific Analysis of Seventh-Century Glass Fragments from the Crypta Balbi in Rome. Archaeometry 2000, 42, 359–374. [Google Scholar] [CrossRef]
- Heiden, E.S.; Gore, D.B.; Stark, S.C. Transportable EDXRF analysis of environmental water samples using Amberlite IRC748 ion-exchange preconcentration. X-Ray Spectrom. 2010, 39, 176–183. [Google Scholar] [CrossRef]
- Solé, V.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Keith, L.H.; Crummett, W.; Deegan, J.; Libby, R.A.; Taylor, J.K.; Wentler, G. Principles of environmental analysis. Anal. Chem. 1983, 55, 2210–2218. [Google Scholar] [CrossRef]
- European Synchrotron Radiation Facility (ESRF), PyMca5 5.6.7 Documentation, Tutorials and Exercises. Available online: https://www.silx.org/doc/PyMca/latest/training/quantification/index.html#id9 (accessed on 22 July 2022).
- US Environmental Protection Agency. Method 6200: Field portable X-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment. In Test Methods for Evaluating Solid Waste; US Environmental Protection Agency: Washington, DC, USA, 2007; pp. 14–15. [Google Scholar]
- Kadachi, A.N.; Al-Eshaikh, M.A. Limits of detection in XRF spectroscopy. X-Ray Spectrom. 2012, 41, 350–354. [Google Scholar] [CrossRef]
- Rousseau, R.M.; Willis, J.P.; Duncan, A.R. Practical XRF calibration procedures for major and trace elements. X-Ray Spectrom. 1996, 25, 179–189. [Google Scholar] [CrossRef]
- Baxter, M.J. Multivariate Analysis of Data on Glass Compositions: A Methodological Note. Archaeometry 1989, 31, 45–53. [Google Scholar] [CrossRef]
- Renda, V.; Nardo, V.M.; Anastasio, G.; Caponetti, E.; Vasi, C.; Saladino, M.; Armetta, F.; Trusso, S.; Ponterio, R. A multivariate statistical approach of X-ray fluorescence characterization of a large collection of reverse glass paintings. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105655. [Google Scholar] [CrossRef]
- Ganio, M.; Gulmini, M.; Latruwe, K.; Vanhaecke, F.; Degryse, P. Isotopic composition of Sasanian glass found at Veh Ardashir. In Proceedings of the 19th Congress of the Association Internationale Pour l’Histoire du Verre, Piran, Slovenia, 16–22 September 2012. [Google Scholar]
- Rehren, T.; Freestone, I.C. Ancient glass: From kaleidoscope to crystal ball. J. Archaeol. Sci. 2015, 56, 233–241. [Google Scholar] [CrossRef] [Green Version]








| RMs | K | Ca | Ti | Mn | Fe | Co | Ni | Cu | Zn | Sr | Zr | Sn | Sb | Ba | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMOG_A | 2.38 | 3.60 | 0.47 | 0.77 | 0.76 | 0.134 | 0.02 | 0.94 | 0.035 | 0.09 | 0.004 | 0.150 | 1.32 | 0.41 | 0.067 |
| CMOG_B | 0.83 | 6.12 | 0.053 | 0.194 | 0.238 | 0.036 | 0.078 | 2.13 | 0.153 | 0.016 | 0.019 | 0.019 | 0.346 | 0.069 | 0.57 |
| CMOG_D | 9.4 | 10.6 | 0.228 | 0.43 | 0.36 | 0.018 | 0.039 | 0.304 | 0.080 | 0.048 | 0.009 | 0.079 | 0.73 | 0.261 | 0.224 |
| VA_08 | 2.70 | 3.64 | 0.066 | 0.026 | 0.48 | N.D. | 0.002 | N.D. | N.D. | 0.044 | 0.005 | N.D. | N.D. | 0.009 | N.D. |
| VA_27 | 3.49 | 4.81 | 0.114 | 0.036 | 1.09 | 0.001 | 0.006 | N.D. | N.D. | 0.039 | 0.010 | 0.001 | N.D. | 0.010 | N.D. |
| VA_70 | 2.19 | 4.90 | 0.019 | 0.209 | 0.182 | N.D. | 0.001 | 0.004 | N.D. | 0.041 | N.D. | N.D. | N.D. | 0.009 | 0.003 |
| CB_65 | 1.06 | 5.1 | 0.072 | 0.42 | 2.11 | N.D. | 0.003 | 1.20 | 0.007 | 0.041 | 0.006 | 0.110 | 0.51 | 0.021 | 3.13 |
| CB_36 | 0.51 | 4.80 | 0.060 | 0.71 | 1.53 | 0.004 | N.D. | 1.66 | 0.040 | 0.044 | 0.006 | N.D. | 0.075 | 0.029 | 0.139 |
| ID | Hardware | Acquisition Parameters a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Device | Anode, V (max), I (max) | Beam Focusing, Focal Spot | Detector: Type, Active Area, Thickness | Resolution at Mn Kα | CPU Pulse Processing Channels | Spot Focusing Device(s) | Filter, Thickness | Time b | V | I |
| ELIO | Rh 50 kV 50 µA | Pin hole 1.2 mm | SDD 25 mm2 500 µm | 140 eV | 2048 | Laser + Camera | none | 90 s | 40 keV | 40 µA |
| Unisantis | Mo, 50 kV 1000 µA | Polycapillary 80 µm | Si-PIN 7 mm2 300 µm; | 186 eV | 2048 | Laser + Microscope | none | 150 s | 50 keV | 300 µA |
| Frankie | W 50 kV 200 µA | Policapillary 300 µm | SDD 20 mm2 450 µm | 173 eV | 4096 | Laser | none | 200 s | 40 keV | 80 µA |
| NITON | Ag 50 kV 100 µA | Pin hole 3–8 mm | SDD 25 mm2 500 µm | 185 eV | 4096 | Camera | Cu 125 µm | 30 s | 40 keV | 50 µA |
| Fe/Al 125 µm | 30 s | 20 keV | 100 µA | |||||||
| none | 30 s | 8 keV | 100 µA | |||||||
| Element | ELIO | Unisantis | Frankie | NITON | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Correction | Pearson Correlation | Relative Error 2σ, % | Type of Correction | Pearson Correlation | Relative Error 2σ, % | Type of Correction | Pearson Correlation | Relative Error 2σ, % | Type of Correction | Pearson Correlation | Relative Error 2σ, % | |
| K | L | 0.990 | 54.5 | L | 0.996 | 27.4 | L | 0.998 | 34.0 | L | 0.993 | 35.7 |
| Ca | L | 0.946 | 15.8 | L | 0.987 | 13.2 | L | 0.932 | 19.7 | L | 0.905 | 19.5 |
| Ti | L | 0.994 | 32.1 | R | 0.983 | N/A | L | 0.995 | 24.3 | R | 0.983 | N/A |
| Mn | L | 0.993 | 12.4 | L | 0.978 | 15.5 | L | 0.988 | 36.7 | L | 0.974 | 23.7 |
| Fe | L | 0.986 | 33.6 | L | 0.989 | 6.5 | L | 0.994 | 27.4 | L | 0.977 | 33.6 |
| Co | R | 0.989 | N/A | R | 0.992 | N/A | R | 0.999 | N/A | R | N/A | N/A |
| Ni | R | 0.986 | N/A | R | 0.983 | N/A | L | 0.998 | 12.0 | N/A | N/A | N/A |
| Cu | L | 0.970 | 22.7 | L | 0.965 | 49.6 | L | 0.989 | 11.5 | L | 0.977 | 21.0 |
| Zn | L | 0.969 | 22.7 | L | 0.998 | 6.6 | L | 0.998 | 11.9 | R | N/A | N/A |
| Sr | L | 0.969 | 33.0 | L | 0.965 | 11.8 | L | 0.868 | 17.6 | L | 0.944 | 12.9 |
| Zr | R | N/A | N/A | R | N/A | N/A | N/A | N/A | N/A | R | N/A | N/A |
| Sn | R | 0.876 | N/A | R | N/A | N/A | R | 0.846 | N/A | R | 0.983 | N/A |
| Sb | L | 0.983 | 100.0 | R | 0.914 | N/A | L | 0.736 | 64.1 | L | 0.978 | 27.9 |
| Ba | R | N/A | N/A | R | N/A | N/A | R | 0.999 | N/A | N/A | N/A | N/A |
| Pb | L | 1.000 | 33.4 | L | 0.999 | 26.4 | L | 0.995 | 98.0 | L | 1.000 | 20.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsuk, O.; Ferretti, M.; Gorghinian, A.; Fiocco, G.; Malagodi, M.; Agostino, A.; Gulmini, M. Data from Multiple Portable XRF Units and Their Significance for Ancient Glass Studies. Molecules 2022, 27, 6068. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27186068
Yatsuk O, Ferretti M, Gorghinian A, Fiocco G, Malagodi M, Agostino A, Gulmini M. Data from Multiple Portable XRF Units and Their Significance for Ancient Glass Studies. Molecules. 2022; 27(18):6068. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27186068
Chicago/Turabian StyleYatsuk, Oleh, Marco Ferretti, Astrik Gorghinian, Giacomo Fiocco, Marco Malagodi, Angelo Agostino, and Monica Gulmini. 2022. "Data from Multiple Portable XRF Units and Their Significance for Ancient Glass Studies" Molecules 27, no. 18: 6068. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27186068