Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658
Abstract
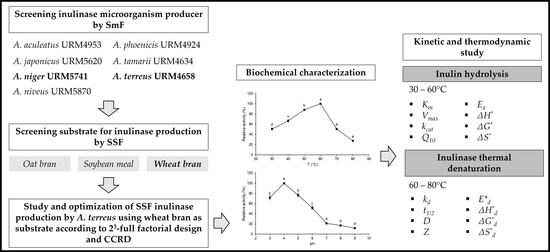
:1. Introduction
2. Results
2.1. Inulinase Production and Optimization
2.2. Biochemical Characterization
2.3. Kinetic and Thermodynamic Parameters of Inulin Hydrolysis
2.4. Kinetic and Thermodynamic Parameters of Inulinase Thermal Denaturation
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Inoculum Preparation
4.2. Screening of Inulinase Production by Submerged Fermentation
4.3. Production of Inulinase by Solid-State Fermentation
4.4. Analytical Determinations
4.4.1. Enzyme Assays
4.4.2. Protein Determination
4.5. Biochemical Characterization
4.5.1. Effect of Temperature and pH on Inulinase Activity
4.5.2. Effect of Metal Ions on Inulinase Activity
4.6. Kinetic/Thermodynamic Study
4.6.1. Kinetic and Thermodynamic Parameters of Inulin Hydrolysis
4.6.2. Kinetic and Thermodynamic Parameters of Inulinase Thermal Denaturation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, R.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bhat, R.; Selvaraj, R. Review of inulinase production using solid-state fermentation. Ann. Microbiol. 2019, 69, 201–209. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. A panorama of bacterial inulinases: Production, purification, characterization and industrial applications. Int. J. Biol. Macromol. 2017, 96, 312–322. [Google Scholar] [CrossRef]
- Paul, I.; Kumar, C.G. Fungal biofactories as potential inulinase sources for production of fructooligosaccharides. In New and Future Developments in Microbial Biotechnology and Bioengineering, 1st ed.; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–210. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int. J. Biol. Macromol. 2016, 85, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.-M.; Zhang, T.; Cao, T.-S.; Liu, X.-Y.; Cui, W.; Zhao, C.-H. Biotechnological potential of inulin for bioprocesses. Bioresour. Technol. 2011, 102, 4295–4303. [Google Scholar] [CrossRef]
- Singh, R. Inulinases. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Pandey, A., Nagi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 423–446. [Google Scholar] [CrossRef]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.D.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Ohara, A.; Nishide, T.G.; Albernaz, J.R.M.; Soares, M.H.; Sato, H.H. A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatal. Agric. Biotechnol. 2015, 4, 199–207. [Google Scholar] [CrossRef]
- Silva, J.D.C.; de França, P.R.L.; Converti, A.; Porto, T.S. Kinetic and thermodynamic characterization of a novel Aspergillus aculeatus URM4953 polygalacturonase. Comparison of free and calcium alginate-immobilized enzyme. Process Biochem. 2018, 74, 61–70. [Google Scholar] [CrossRef]
- Gohel, V.; Naseby, D. Thermalstabilization of chitinolytic enzymes of Pantoea dispersa. Biochem. Eng. J. 2007, 35, 150–157. [Google Scholar] [CrossRef]
- Souza, P.M.; Aliakbarian, B.; Filho, E.X.F.; Magalhães, P.O.; Junior, A.P.; Converti, A.; Perego, P. Kinetic and thermodynamic studies of a novel acid protease from Aspergillus foetidus. Int. J. Biol. Macromol. 2015, 81, 17–21. [Google Scholar] [CrossRef]
- El Aty, A.A.A.; Wehaidy, H.R.; Mostafa, F.A. Optimization of inulinase production from low cost substrates using Plackett–Burman and Taguchi methods. Carbohydr. Polym. 2014, 102, 261–268. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; da Silva, M.F.; Converti, A.; Porto, T.S. Production of β-fructofuranosidase with transfructosylating activity by Aspergillus tamarii URM4634 Solid-State Fermentation on agroindustrial by-products. Int. J. Biol. Macromol. 2020, 144, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Correction: Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 75. [Google Scholar] [CrossRef]
- Converti, A.; Pessoa, A.; Silva, J.D.C.; de Oliveira, R.L.; Porto, T.S. Thermodynamics Applied to Biomolecules. In Pharmaceutical Biotechnology, 1st ed.; Pessoa, A., Vitolo, M., Long, P.F., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 29–42. [Google Scholar] [CrossRef]
- Xiong, C.; Jinhua, W.; Dongsheng, L. Optimization of solid-state medium for the production of inulinase by Kluyveromyces S120 using response surface methodology. Biochem. Eng. J. 2007, 34, 179–184. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Jindal, A. Response surface optimization of solid state fermentation for inulinase production from Penicillium oxalicum using corn bran. J. Food Sci. Technol. 2018, 55, 2533–2540. [Google Scholar] [CrossRef]
- Rawat, H.K.; Soni, H.; Suryawanshi, R.K.; Choukade, R.; Prajapati, B.P.; Kango, N. Exo-inulinase production from Aspergillus fumigatus NFCCI 2426: Purification, characterization, and immobilization for continuous fructose production. J. Food Sci. 2021, 86, 1778–1790. [Google Scholar] [CrossRef]
- Germec, M.; Turhan, I. Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioprocess Biosyst. Eng. 2019, 42, 1993–2005. [Google Scholar] [CrossRef]
- Trivedi, S.; Divecha, J.; Shah, A. Optimization of inulinase production by a newly isolated Aspergillus tubingensis CR16 using low cost substrates. Carbohydr. Polym. 2012, 90, 483–490. [Google Scholar] [CrossRef]
- Yewale, T.; Singhal, R.S.; Vaidya, A.A. Immobilization of inulinase from Aspergillus niger NCIM 945 on chitosan and its application in continuous inulin hydrolysis. Biocatal. Agric. Biotechnol. 2013, 2, 96–101. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Ma, W.; Yang, J.-M.; Kang, Y.; Park, Y.-D. Effects of Cu2+ on alkaline phosphatase from Macrobrachium rosenbergii. Int. J. Biol. Macromol. 2018, 117, 116–123. [Google Scholar] [CrossRef]
- Li, J.; Lock, R.A.; Klaren, P.; Swarts, H.G.; Stekhoven, F.M.; Bonga, S.E.; Flik, G. Kinetics of Cu2+ inhibition of Na+K+-ATPase. Toxicol. Lett. 1996, 87, 31–38. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Covalent immobilization and thermodynamic characterization of pullulanase for the hydrolysis of pullulan in batch system. Carbohydr. Polym. 2010, 81, 252–259. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Zhu, D.; Ohta, Y.; Wang, Y. Purification and characterization of inulinase from Aspergillus niger AF10 expressed in Pichia pastoris. Protein Expr. Purif. 2004, 35, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Rawat, H.K.; Jain, S.C.; Kango, N. Production and properties of inulinase from Penicillium sp. NFCC 2768 grown on inulin-rich vegetal infusions. Biocatal. Biotransformation 2015, 33, 61–68. [Google Scholar] [CrossRef]
- Abu El-Souod, S.M.; Mohamed, T.; Ali, E.M.; El-Badry, M.O.; El-Keiy, M.M. Partial purification of extracellular exo-inulinase from Ulocladium atrum. J. Genet. Eng. Biotechnol. 2014, 12, 15–20. [Google Scholar] [CrossRef]
- Coitinho, J.B.; Guimarães, V.M.; de Almeida, M.N.; Falkoski, D.L.; de Queiróz, J.H.; de Rezende, S.T. Characterization of an exoinulinase produced by Aspergillus terreus CCT 4083 grown on sugar cane bagasse. J. Agric. Food Chem. 2010, 58, 8386–8391. [Google Scholar] [CrossRef]
- Miłek, J. Application of the new method to determine the activation energies and optimum temperatures of inulin hydrolysis by exo-inulinases Aspergillus niger. J. Therm. Anal. 2022, 147, 1371–1377. [Google Scholar] [CrossRef]
- Saleh, S.A.; El-Galil, A.A.A.; Sakr, E.A.; Taie, H.A.; Mostafa, F.A. Physiochemical, kinetic and thermodynamic studies on Aspergillus wewitschiae MN056175 inulinase with extraction of prebiotic and antioxidant Cynara scolymus leaves fructo-oligosaccharides. Int. J. Biol. Macromol. 2020, 163, 1026–1036. [Google Scholar] [CrossRef]
- Miłek, J. The inulin hydrolysis by recombinant exo-inulinases: Determination the optimum temperatures and activation energies. J. Therm. Anal. 2022, 147, 8061–8067. [Google Scholar] [CrossRef]
- Awad, G.; Wehaidy, H.R.; El Aty, A.A.; Hassan, M.E. A novel alginate–CMC gel beads for efficient covalent inulinase immobilization. Colloid Polym. Sci. 2017, 295, 495–506. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Silva, M.F.; Converti, A.; Porto, T.S. Biochemical characterization and kinetic/thermodynamic study of Aspergillus tamarii URM4634 β-fructofuranosidase with transfructosylating activity. Biotechnol. Prog. 2019, 35, e2879. [Google Scholar] [CrossRef]
- Riaz, M.; Perveen, R.; Javed, M.R.; Nadeem, H.; Rashid, M.H. Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzym. Microb. Technol. 2007, 41, 558–564. [Google Scholar] [CrossRef]
- Wehaidy, H.R.; Abdel-Naby, M.A.; Shousha, W.G.; Elmallah, M.I.; Shawky, M.M. Improving the catalytic, kinetic and thermodynamic properties of Bacillus subtilis KU710517 milk clotting enzyme via conjugation with polyethylene glycol. Int. J. Biol. Macromol. 2018, 111, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. Fructose production from inulin using fungal inulinase immobilized on 3-aminopropyl-triethoxysilane functionalized multiwalled carbon nanotubes. Int. J. Biol. Macromol. 2019, 125, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, T.; Kennedy, J.F. Purification, thermodynamics and kinetic characterization of fungal endoinulinase for the production of fructooligosaccharides from inulin. Int. J. Biol. Macromol. 2020, 164, 3535–3545. [Google Scholar] [CrossRef]
- Karimi, M.; Chaudhury, I.; Jianjun, C.; Safari, M.; Sadeghi, R.; Habibi-Rezaei, M.; Kokini, J. Immobilization of endo-inulinase on non-porous amino functionalized silica nanoparticles. J. Mol. Catal. B Enzym. 2014, 104, 48–55. [Google Scholar] [CrossRef]
- Tayefi-Nas, H.; Asadpour, R. Effect of heat treatment on buffalo (Bubalus bubalis) lactoperoxidase activity in raw milk. J. Biol. Sci. 2008, 8, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Pace, C. Contribution of the hydrophobic effect to globular protein stability. J. Mol. Biol. 1992, 226, 29–35. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Pandey, A.; Larroche, C.; Kennedy, J.F. Purification and characterization of two isoforms of exoinulinase from Penicillium oxalicum BGPUP-4 for the preparation of high fructose syrup from inulin. Int. J. Biol. Macromol. 2018, 118, 1974–1983. [Google Scholar] [CrossRef]
- Karam, E.A.; Wahab, W.A.A.; Saleh, S.A.; Hassan, M.E.; Kansoh, A.L.; Esawy, M.A. Production, immobilization and thermodynamic studies of free and immobilized Aspergillus awamori amylase. Int. J. Biol. Macromol. 2017, 102, 694–703. [Google Scholar] [CrossRef]
- Skowronek, M.; Fiedurek, J. Selection of biochemical mutants of Aspergillus niger resistant to some abiotic stresses with increased inulinase production. J. Appl. Microbiol. 2003, 95, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dixon, M.; Webb, E.C. Enzyme Inhibition and Inactivation. In Enzymes; Dixon, M., Webb, E., Eds.; Longman: London, UK, 1979; pp. 332–467. [Google Scholar]





| Strain | Inulinase Activity (U mL−1) |
|---|---|
| A. aculeatus URM4953 | 0.84 ± 0.12 b,c |
| A. japonicus URM5620 | 0.93 ± 0.12 b,c |
| A. niger URM5741 | 2.20 ± 0.12 a |
| A. niveus URM5870 | 0.63 ± 0.18 c |
| A. phoenicis URM4924 | 0.84 ± 0.11 b,c |
| A. tamarii URM4634 | 0.68 ± 0.05 c |
| A. terreus URM4658 | 1.27 ± 0.23 b |
| Substrate | Inulinase Activity (U mL−1) | |
| A. niger URM5741 | A. terreus URM4658 | |
| Oat bran | 5.57 ± 0.36 c | 10.26 ± 0.05 c |
| Soybean meal | 10.41 ± 0.87 a | 11.83 ± 0.15 b |
| Wheat bran | 8.51 ± 0.92 b | 13.34 ± 0.41 a |
| Run | Substrate Amount (g) | Inulin Concentration (%) | Moisture Content (%) | Inulinase Activity (U mL−1) |
|---|---|---|---|---|
| 1 | 3 | 2.5 | 50 | 10.97 |
| 2 | 7 | 2.5 | 50 | 10.54 |
| 3 | 3 | 7.5 | 50 | 14.35 |
| 4 | 7 | 7.5 | 50 | 11.23 |
| 5 | 3 | 2.5 | 70 | 11.26 |
| 6 | 7 | 2.5 | 70 | 9.27 |
| 7 | 3 | 7.5 | 70 | 12.57 |
| 8 | 7 | 7.5 | 70 | 10.14 |
| 9 | 5 | 5.0 | 60 | 13.62 |
| 10 | 5 | 5.0 | 60 | 13.22 |
| 11 | 5 | 5.0 | 60 | 13.12 |
| Run | Inulin Concentration (%) | Moisture Content (%) | Inulinase Activity (U mL−1) |
|---|---|---|---|
| 1 | 6.0 | 45 | 11.30 |
| 2 | 6.0 | 55 | 12.97 |
| 3 | 9.0 | 45 | 11.63 |
| 4 | 9.0 | 55 | 15.08 |
| 5 | 5.4 | 50 | 14.68 |
| 6 | 9.6 | 50 | 13.91 |
| 7 | 7.5 | 43 | 10.72 |
| 8 | 7.5 | 57 | 13.37 |
| 9 | 7.5 | 50 | 12.61 |
| 10 | 7.5 | 50 | 12.75 |
| 11 | 7.5 | 50 | 12.97 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value a |
|---|---|---|---|---|---|
| (1) Inulin concentration (L) | 0.22 | 1 | 0.22 | 6.86 | 0.120 |
| Inulin concentration (Q) | 2.41 | 1 | 2.40 | 72.06 | 0.013 |
| (2) Moisture content (L) | 9.83 | 1 | 9.83 | 294.33 | 0.003 |
| Moisture content (Q) | 1.26 | 1 | 1.26 | 37.79 | 0.025 |
| 1 (L) × 2 (L) | 0.79 | 1 | 0.79 | 23.69 | 0.040 |
| Lack of fit | 2.14 | 3 | 0.71 | 21.35 | 0.045 |
| Pure error | 0.06 | 2 | 0.03 | ||
| Total SS | 18.19 | 10 |
| Metal Ion | Residual Inulinase Activity (%) |
|---|---|
| Ca2+ | 98.31 ± 0.10 a |
| Cu2+ | 64.59 ± 0.27 d |
| Fe2+ | 92.40 ± 0.45 a,b |
| Hg2+ | 14.79 ± 1.50 e |
| K+ | 87.84 ± 2.26 b,c |
| Mg2+ | 90.34 ± 1.21 a,b |
| Na+ | 79.91 ± 4.76 c |
| Zn2+ | 86.59 ± 2.78 b,c |
| Parameter | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 30 | 40 | 50 | 55 | 60 | |
| aKm (mM) | 0.78 ± 0.03 C | 0.86 ± 0.10 C | 1.68 ± 0.08 B | 1.72 ± 0.05 A,B | 2.02 ± 0.12 A |
| bVmax (mM min−1) | 13.09 ± 0.20 C | 16.03 ± 0.89 C | 26.46 ± 0.29 B | 30.30 ± 0.36 B | 35.09 ± 1.57 A |
| ckcat (min−1) | 2.49 ± 0.04 C | 3.05 ± 0.17 C | 5.03 ± 0.06 B | 5.76 ± 0.07 B | 6.68 ± 0.30 A |
| R2 | 0.939 | 0.929 | 0.964 | 0.971 | 0.974 |
| dQ10 | 1.08 ± 0.004 A | 1.08 ± 0.004 A | 1.08 ± 0.004 A | 1.07 ± 0.004 A | 1.07 ± 0.004 A |
| e ΔH* (kJ mol−1) | 17.07 ± 1.10 A | 16.99 ± 1.10 A | 16.90 ± 1.10 A | 16.86 ± 1.10 A | 16.82 ± 1.10 A |
| f ΔG* (kJ mol−1) | 82.31 ± 0.03 E | 84.58 ± 0.14 D | 86.02 ± 0.03 C | 87.02 ± 0.03 B | 87.98 ± 0.12 A |
| g ΔS* (J K−1 mol−1) | −215.20 ± 3.76 A | −215.85 ± 3.06 A | −213.88 ± 3.31 A | −213.80 ± 3.46 A | −213.60 ± 3.68 A |
| Parameter | T (°C) | ||||
|---|---|---|---|---|---|
| 60 | 65 | 70 | 75 | 80 | |
| akd (min−1) | 0.0013 ± 0.0002 | 0.0100 ± 0.0015 | 0.0152 ± 0.0007 | 0.0307 ± 0.0022 | 0.0794 ± 0.0007 |
| R2 | 0.997 | 0.997 | 0.959 | 0.970 | 0.988 |
| bt1/2 (min) | 519.86 ± 81.69 | 70.16 ± 10.91 | 47.21 ± 2.27 | 22.64 ± 1.67 | 8.73 ± 0.08 |
| cD-value (min) | 1726.94 ± 271.36 | 233.08 ± 36.26 | 161.59 ± 7.54 | 75.21 ± 5.54 | 29.00 ± 0.26 |
| dZ-value (°C) | 12.39 ± 0.14 | ||||
| eE*d (kJ mol−1) | 182.18 ± 2.11 | ||||
| f ΔG*d (kJ mol−1) | 111.56 ± 0.44 | 107.64 ± 0.44 | 108.25 ± 0.01 | 107.65 ± 0.21 | 106.44 ± 0.03 |
| g ΔH*d (kJ mol−1) | 179.40 ± 2.11 | 179.36 ± 2.11 | 179.32 ± 2.11 | 179.28 ± 2.11 | 179.24 ± 2.11 |
| h ΔS*d (J mol−1 K−1) | 203.64 ± 5.02 | 212.08 ± 7.54 | 207.10 ± 6.11 | 205.73 ± 5.45 | 206.11 ± 6.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, R.L.; da Silva, S.P.; Converti, A.; Porto, T.S. Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658. Molecules 2022, 27, 6418. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27196418
de Oliveira RL, da Silva SP, Converti A, Porto TS. Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658. Molecules. 2022; 27(19):6418. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27196418
Chicago/Turabian Stylede Oliveira, Rodrigo Lira, Suzana Pedroza da Silva, Attilio Converti, and Tatiana Souza Porto. 2022. "Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658" Molecules 27, no. 19: 6418. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27196418