Ent-Abietane Diterpenoids from Euphorbia fischeriana and Their Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
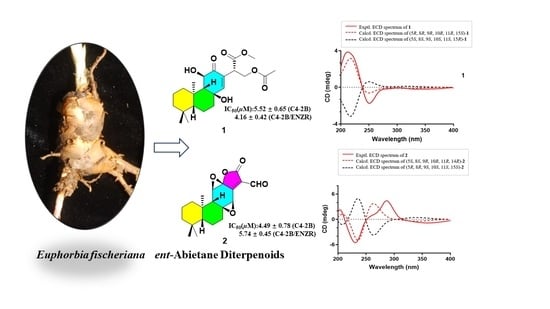
2.1. Structure Elucidation of Compounds 1 and 2
2.2. Biological Activity of Isolated Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Quantum Chemical NMR and ECD Calculations of Compound 1–2
3.5. Cell Culture
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2009, 26, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Zhang, H.; Liu, J. Structural diversity and biological activities of diterpenoids derived from Euphorbia fischeriana steud. Molecules 2018, 23, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, L.; Kong, C.; Mei, W.; Dai, H.; Xu, F.; Huang, S. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sonwal, S.; Hwang, S.-K.; Shukla, S.; Khan, I.; Dey, D.K.; Chen, L.; Simal-Gandara, J.; Xiao, J.; Huh, Y.S.; et al. Sugiol, a diterpenoid: Therapeutic actions and molecular pathways involved. Pharmacol. Res. 2021, 163, 105313. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.C.; Dao, D.Q.; Mai, T.V.T.; Nguyen, T.L.A.; Huynh, L.K. On the radical scavenging and DNA repairing activities by natural oxygenated diterpenoids: Theoretical insights. J. Chem. Inf. Model. 2022, 62, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-M.; Tsai, C.-H.; Yang, Y.-C.; Tu, W.-C.; Chen, L.-R.; Liang, Y.-S.; Wang, S.-Y.; Shyur, L.-F.; Chien, S.-C.; Cha, T.-L.; et al. A novel diterpene suppresses CWR22Rv1 tumor growth in vivo through antiproliferation and proapoptosis. Cancer Res. 2008, 68, 6634–6642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 298. [Google Scholar]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-L.; Zhang, J.-S.; Huang, J.-L.; Zhang, Y.; Chen, J.-Q.; Tang, G.-H.; Yin, S. Euphonoids A−G, cytotoxic diterpenoids from Euphorbia fischeriana. Phytochemistry 2019, 166, 112064. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, T.; Bittner, M.; Silva, M.; Aqueveque, P.; Jakupovic, J. Diterpenes and phloracetophenones from Euphorbia portulacoides. Phytochemistry 1996, 41, 1149–1153. [Google Scholar] [CrossRef]
- Uemura, D.; Hirata, Y. Two new diterpenoids, jolkinolides A and B, obtained from Euphorbia jolkini boiss. (Euphorbiaceae). Tetrahedron Lett. 1972, 13, 1387–1390. [Google Scholar] [CrossRef]
- Liu, G.-L.; Xu, W.; Liu, X.-J.; Yan, X.-L.; Chen, J. Two new abietane diterpenoids from the leaves of Rabdosia serra. J. Asian Nat. Prod. Res. 2018, 22, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.-Y.; Zhang, H.; Han, C.-C.; Liu, J.-C. Anti-cancer activities of diterpenoids derived from Euphorbia fischeriana Steud. Molecules 2018, 23, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosaria, A.; Giuseppe, A.M.; Monica, R.L.; Xiao, J.-B.; Simone, B.; Rosa, T. Advances on natural abietane, labdane and clerodane diterpenes as anti-cancer agents: Sources and mechanisms of action. Molecules 2022, 27, 4791. [Google Scholar]
- Yan, X.-L.; Zou, M.-F.; Chen, B.-L.; Yuan, F.-Y.; Zhu, Q.-F.; Zhang, X.; Lin, Y.; Long, Q.-D.; Liu, W.-L.; Liao, S.-G. Euphorane C, an unusual C17-norabietane diterpenoid from Euphorbia dracunculoides induces cell cycle arrest and apoptosis in human leukemia K562 cells. Arab. J. Chem. 2022, 15, 104203. [Google Scholar] [CrossRef]
- Sang, J.; Li, W.; Diao, H.-J.; Fan, R.-Z.; Huang, J.-L.; Gan, L.; Zou, M.-F.; Tang, G.-H.; Yin, S. Jolkinolide B targets thioredoxin and glutathione systems to induce ROS-mediated paraptosis and apoptosis in bladder cancer cells. Cancer Lett. 2021, 509, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Gan, L.; Zou, M.-F.; Lin, Z.-J.; Fan, R.-Z.; Huang, J.-L.; Li, W.; Tang, G.-H.; Yin, S. Jolkinolide B sensitizes bladder cancer to mTOR inhibitors via dual inhibition of Akt signaling and autophagy. Cancer Lett. 2022, 526, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.-Q.; Yan, S.-S.; Shen, S.-S.; Zhu, H.-L.; Gu, Y.; Wang, H.-B.; Qin, G.-W.; Yu, Q. 17-hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus kinases and induces apoptosis of human cancer cells. Cancer Res. 2009, 69, 7302–7310. [Google Scholar] [CrossRef] [PubMed]

 ) and HMBC (
) and HMBC ( ) correlations of 1 and 2.
) correlations of 1 and 2.
 ) of compounds 1 and 2.
) of compounds 1 and 2.



| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Type | δH (J in Hz) | Δc, Type | |
| 1 | 1.88 (1H, d, J = 13.0 Hz) 1.21–1.16 (1H, m) | 39.7, CH2 | 1.92 (1H, d, J = 12.5 Hz) 1.37–1.29 (1H, m) | 39.2, CH2 |
| 2 | 1.55 (1H, d, J = 13.5 Hz) 1.51–1.45 (1H, m) | 18.5, CH2 | 1.63–1.55 (1H, m) 1.56–1.51 (1H, m) | 18.5, CH2 |
| 3 | 1.43 (1H, d, J = 12.7 Hz) 1.26–1.19 (1H, m) | 41.6, CH2 | 1.47–1.43 (1H, m) 1.29–1.20 (1H, m) | 41.4, CH2 |
| 4 | 33.3, C | 33.6, C | ||
| 5 | 1.08 (1H, dd, J = 12.9, 2.6 Hz) | 54.4, CH | 1.12 (1H, dd, J = 12.3, 2.5 Hz) | 53.6, CH |
| 6 | 1.71–1.65 (1H, m) 1.16–1.12 (1H, m) | 19.2, CH2 | 1.88–1.80 (1H, m) 1.56–1.51 (1H, m), overlapped | 20.9, CH2 |
| 7 | 2.13–2.03(1H, m) 1.74–1.72 (1H, m) | 41.4, CH2 | 2.08–1.97 (1H, m) 1.51–1.48 (1H, m), overlapped | 35.8, CH2 |
| 8 | 69.5, C | 71.7, C | ||
| 9 | 2.13–2.03(1H, s) | 60.4, CH | 2.33 (1H, s) | 48.1, CH |
| 10 | 37.7, C | 39.4, C | ||
| 11 | 4.23 (1H, s) | 72.0, CH | 4.15 (1H, s) | 64.8, CH |
| 12 | 196.0, C | 85.4, C | ||
| 13 | 133.7, C | 166.2, C | ||
| 14 | 6.71 (1H, s) | 154.2, CH | 4.48 (1H, s) | 55.3, CH |
| 15 | 3.77 (1H, t, J = 7.2 Hz) | 45.7, CH | 127.5, C | |
| 16 | 170.8, C | 165.8, C | ||
| 17 | 4.41 (2H, d, J = 7.2 Hz) | 62.5, CH2 | 9.97 (1H, s) | 185.0, CH |
| 18 | 0.91 (3H, s) | 34.0, CH3 | 0.94 (3H, s) | 33.6, CH3 |
| 19 | 0.79 (3H, s) | 22.1, CH3 | 0.85 (3H, s) | 22.0, CH3 |
| 20 | 0.69 (3H, s) | 17.8, CH3 | 0.80 (3H, s) | 15.6, CH3 |
| 16-OCH3 | 3.68 (3H, s) | 52.4, CH3 | ||
| 1′ | 171.1, C | |||
| 2′ | 2.02 (3H, s) | 21.0, CH3 | ||
| Compound | IC50 1 (µM) | ||||
|---|---|---|---|---|---|
| MDA-MB-231 | HCT-15 | RKO | C4-2B | C4-2B/ENZR | |
| 1 | 21.80 ± 2.35 | 28.57 ± 1.16 | 20.46 ± 1.43 | 5.52 ± 0.65 | 4.16 ± 0.42 |
| 2 | 7.95 ± 0.82 | 12.45 ± 3.24 | 8.78 ± 2.45 | 4.49 ± 0.78 | 5.74 ± 0.45 |
| 3 | >50 | >50 | >50 | 34.09 ± 7.78 | >50 |
| 4 | >50 | >50 | >50 | 23.34 ± 2.18 | 36.98 ± 6.18 |
| DOX 2 | 0.34 ± 0.16 | 0.72 ± 0.09 | 0.59 ± 0.29 | 0.11 ± 0.08 | 0.22 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.-F.; Xu, G.-B.; Liao, S.-G.; Yan, X.-L. Ent-Abietane Diterpenoids from Euphorbia fischeriana and Their Cytotoxic Activities. Molecules 2022, 27, 7258. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217258
Zhu Q-F, Xu G-B, Liao S-G, Yan X-L. Ent-Abietane Diterpenoids from Euphorbia fischeriana and Their Cytotoxic Activities. Molecules. 2022; 27(21):7258. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217258
Chicago/Turabian StyleZhu, Qin-Feng, Guo-Bo Xu, Shang-Gao Liao, and Xue-Long Yan. 2022. "Ent-Abietane Diterpenoids from Euphorbia fischeriana and Their Cytotoxic Activities" Molecules 27, no. 21: 7258. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217258