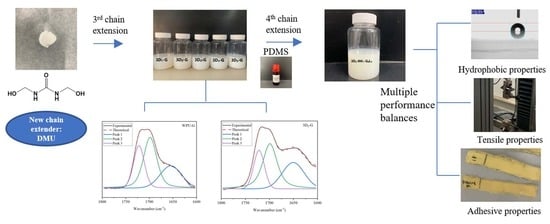
The Multi-Step Chain Extension for Waterborne Polyurethane Binder of Para-Aramid Fabrics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chain Extension by DMU
2.2. Impact of DMU Content on WPU
2.3. Influence of CE-3 Types
2.4. Influence of the Addition Sequence of DMU
2.5. Influence of the Macrodiol
2.6. Properties as Fabric Adhesives
3. Materials and Methods
3.1. Materials
3.2. Preparation of WPU Dispersion
3.3. Film Formation
3.4. Adhesive Joint Preparation
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, R.; Cao, H.; Zhang, H.; Qiao, L.; Wang, F.; Wang, X. Terminal hydrophilicity-induced dispersion of cationic waterborne polyurethane from CO2-based polyol. Macromolecules 2020, 53, 6322–6330. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Saralegi, A.; Costa, M.; Barreiro, F.; Corcuera, M.; Eceiza, A. Synthesis of waterborne polyurethane-urea dispersions with chain extension step in homogeneous and heterogeneous media. J. Colloid Interf. Sci. 2016, 476, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xu, J.; Li, J.; Qiu, H.; Zheng, S.; Yang, J. A crosslinkable graphene oxide in waterborne polyurethane anticorrosive coatings: Experiments and simulation. Compos. Part B-Eng. 2020, 188, 10788. [Google Scholar] [CrossRef]
- Yang, D.; Han, L.; Zhang, H.; Qiu, F. Monocomponent waterborne polyurethane adhesives: Influence of the crosslinking agent on their properties. J. Macromol. Sci. A 2011, 48, 277–283. [Google Scholar] [CrossRef]
- Fuensanta, M.; Jofre-Reche, J.; Rodríguez-Llansolab, F.; Costa, V.; Martín-Martínez, J. Structure and adhesion properties before and after hydrolytic ageing of polyurethane urea adhesives made with mixtures of waterborne polyurethane dispersions. Int. J. Adhes. Adhes. 2018, 85, 165–176. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Liu, L.; Lu, K.; Li, G.; Yang, X. Two-stage interface enhancement of aramid fiber composites: Establishment of hierarchical interphase with waterborne polyurethane sizing and oxazolidone-containing epoxy matrix. J. Compos. Sci. Technol. 2020, 193, 108114. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Liang, L.; Yang, M.; Wang, S.; Shi, H.; Xie, Y.; Pi, K. Application of polyether amine intercalated graphene oxide as filler for enhancing hydrophobicity, thermal stability, mechanical and anti-corrosion properties of waterborne polyurethane. Diam. Relat. Mater. 2020, 109, 108077. [Google Scholar] [CrossRef]
- Pan, P.; Du, X.; Qiu, J.; Du, Z.; Wang, H.; Cheng, X. Excellent mechanical and transparency properties of cationic waterborne polyurethane films modified by boehmite sol. J. Macromol. Mater. Eng. 2020, 305, 2000021. [Google Scholar] [CrossRef]
- Yu, F.; Cao, L.; Meng, Z.; Lin, N.; Liu, X. Crosslinked waterborne polyurethane with high waterproof performance. J. Polym. Chem. 2016, 7, 3913–3922. [Google Scholar] [CrossRef]
- Lyu, J.; Xu, K.; Zhang, N.; Lu, C.; Zhang, Q.; Yu, L.; Feng, F.; Li, N. In situ incorporation of diamino silane group into waterborne polyurethane for enhancing surface hydrophobicity of coating. Molecules 2019, 24, 1667. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Z.; Shi, S.; Ren, J.; Dong, X. Environmentally friendly plant-based waterborne polyurethane for hydrophobic and heat-resistant films. J. Appl. Polym. Sci. 2022, 139, e52437. [Google Scholar] [CrossRef]
- García, J.; García, F.; Serna, F.; Peña, J. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Zhang, B.; Jia, L.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Surface and interface modification of aramid fiber and its reinforcement for polymer composites: A review. Eur. Polym. J. 2021, 147, 11035. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, H.; Chen, P.; Zhang, Z.; Hu, C. Preparation and properties of waterborne polyurethane with star-shaped hyperbranched structure. Polymer 2019, 180, 121690. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Qiu, T.; Xiao, W.; Du, D.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films. J. Appl. Surf. Sci. 2016, 377, 66–74. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. J. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef] [Green Version]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Retegi, A.; Arbelaiz, A.; Corcuera, M.; Eceiza, A. Development of waterborne polyurethane-ureas added with plant extracts: Study of different incorporation routes and their influence on particle size, thermal, mechanical and antibacterial properties. Prog. Org. Coat. 2018, 117, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Li, S.; Huang, H.; Zhao, Y.; Chen, Y.; Zhang, L.; Xie, D. Anticorrosive and UV-blocking waterborne polyurethane composite coating containing novel two-dimensional Ti3C2 MXene nanosheets. J. Mater Sci. 2020, 56, 4212–4224. [Google Scholar] [CrossRef]
- Liang, H.; Wang, S.; He, H.; Wang, M.; Liu, L.; Lu, J.; Zhang, Y.; Zhang, C. Aqueous anionic polyurethane dispersions from castor oil. Ind. Crop. Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-bonding cross-linked supramolecular polymeric materials as substrates for stretchable, antitearing, and self-healable thin film electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef]
- Fan, W.; Jin, Y.; Shi, L.; Du, W.; Zhou, R.; Lai, S.; Shen, Y.; Li, Y. Simultaneously achieving fast self-healing and reprocessing of supertough water-dispersed “living” supramolecular polymers containing dynamic ditelluride bonds under visible light. ACS Appl. Mater. Interfaces 2020, 12, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhang, S.; Sun, P.; Tang, B.; Yin, Z.; Cao, X.; Chen, Q.; Xu, J.; Zhang, X. Tough and multi-recyclable cross-linked supramolecular polyureas via incorporating noncovalent bonds into main-chains. Adv. Mater. 2020, 32, 2000096. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Xia, Z.; Qi, Y.; Sumigawa, T.; Wu, J.; Šesták, P.; Lu, Y.; Håkonsen, V.; Li, T.; Wang, F.; et al. Simultaneously toughening and stiffening elastomers with octuple hydrogen bonding. Adv. Mater. 2021, 2008523. [Google Scholar] [CrossRef] [PubMed]
- Sijbesma, R.; Beijer, F.; Brunsveld, L.; Folmer, B.; Hirschberg, J.; Lange, R.; Lowe, J.; Meijer, E. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, Y.; Nan, Y.; Okuro, K.; Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, Y.; Park, S.; Lee, Y.; Kim, H. Preparation and properties of waterborne polyurethanes for water-vapor-permeable coating materials. J. Appl. Polym. Sci. 2003, 89, 123–129. [Google Scholar] [CrossRef]
- Ou, C.; Su, C.; Jeng, U.; Hsu, S. Characterization of biodegradable polyurethane nanoparticles and thermally induced self-assembly in water dispersion. ACS Appl. Mater. Interfaces 2014, 6, 5685–5694. [Google Scholar] [CrossRef]
- Meng, Q.; Lee, S.; Nah, C.; Lee, Y. Preparation of waterborne polyurethanes using an amphiphilic diol for breathable waterproof textile coatings. Prog. Org. Coat. 2009, 66, 382–386. [Google Scholar] [CrossRef]
- Cakić, S.; Ristic, I.; Krakovsky, I.; Stojiljkovic, D.; Belsky, P.; Kollova, L. Crystallization and thermal properties in waterborne polyurethane elastomers: Influence of mixed soft segment block. Mater. Chem. Phys. 2014, 144, 31–40. [Google Scholar] [CrossRef]
- Cakić, S.; Ristic, I.; Marinovic-Cincovic, M.; Spirkova, M. The effects of the structure and molecular weight of the macrodiol on the properties polyurethane anionic adhesives. Int. J. Adhes. Adhes. 2013, 41, 132–139. [Google Scholar] [CrossRef]
- Fuensantaa, M.; Jofre-Rechea, J.; Rodríguez-Llansolab, F.; Víctor, C.; José, I.; Martín-Martíneza, J. Structural characterization of polyurethane ureas and waterborne polyurethane urea dispersions made with mixtures of polyester polyol and polycarbonate diol. Prog. Org. Coat. 2017, 112, 141–152. [Google Scholar] [CrossRef]
- Dai, M.; Wang, J.; Zhang, Y. Improving water resistance of waterborne polyurethane coating with high transparency and good mechanical properties. J. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124994. [Google Scholar] [CrossRef]
- Li, Q.; Ye, J.; Qiu, T.; Guo, L.; He, L.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups: The study on spacer linkages. J. Appl. Polym. Sci. 2018, 46628, 1–10. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J. Thermoplastic polyurethane coatings made with mixtures of polyethers of different molecular weights with pressure sensitive adhesion property. Prog. Org. Coat. 2018, 118, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Fuensanta, M.; Martín-Martínez, J. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2019, 88, 81–89. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J. Structural and Viscoelastic Properties of Thermoplastic Polyurethanes Containing Mixed Soft Segments with Potential Application as Pressure Sensitive Adhesives. Polymers 2021, 13, 3097. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, B.; Xu, Z.; Liu, W. An unparalleled H-bonding and ion-bonding crosslinked waterborne polyurethane with super toughness and unprecedented fracture energy. Mater. Horiz. 2021, 8, 2742–2749. [Google Scholar] [CrossRef]
- Koutsoumpis, S.; Ozimek, J.; Raftopoulos, K.; Hebda, E.; Klonos, P.; Papadakis, C.; Pielichowski, K.; Pissis, P. Polyurethanes with POSS pendent on flexible hard segments: Morphology and glass transition. Polymer 2018, 147, 225–236. [Google Scholar] [CrossRef]
- MacKnight, W.; Papadimitrakopoulos, F. Phase behavior and structure in mesogen containing polyurethanes. In Makromolekulare Chemie: Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1993; Volume 69, pp. 41–49. [Google Scholar] [CrossRef]
- Li, J.W.; Tsen, W.; Tsou, C.; Suen, M.; Chiu, C. Synthetic environmentally friendly castor oil based-polyurethane with carbon black as a microphase separation promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef]
- Diao, S.; Zhang, Y.; Zhao, C.; Wang, M.; Yu, J. Preparation of waterborne polyurethane based on different polyols: The effect of structure and crystallinity. J. Polym. Res. 2022, 29, 105. [Google Scholar] [CrossRef]
- Wu, G.; Liu, D.; Chen, J.; Liu, J.; Kong, Z. Preparation and properties of super hydrophobic films from siloxane-modified two-component waterborne polyurethane and hydrophobic nano SiO2. Prog. Org. Coat. 2019, 127, 80–87. [Google Scholar] [CrossRef]











| Sample | IPDI/g | PTMG + PCL/g | (CE-1)/g | (CE-2)/g | (CE-3)/g | (CE-4)/g | TEA/g |
|---|---|---|---|---|---|---|---|
| WPU-G | 8.89 | 20 | 1.59 | 1.11 | / | / | 1.09 |
| 3D1-G | 8.89 | 20 | 1.58 | 0.88 | 0.32 | / | 1.09 |
| 3D2-G | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D3-G | 8.89 | 20 | 1.59 | 0.39 | 0.95 | / | 1.09 |
| 3D4-G | 8.89 | 20 | 1.59 | 0.15 | 1.28 | / | 1.10 |
| 3D5-G | 8.89 | 20 | 1.52 | 0 | 1.52 | / | 1.04 |
| 1D2-G | 8.89 | 20 | 0.63 | 1.59 | 0.63 | / | 1.09 |
| 2D2-G | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3T2-G | 8.89 | 20 | 1.59 | 0.63 | 0.70 | / | 1.09 |
| 3P2-G | 8.89 | 20 | 1.59 | 0.63 | 0.71 | / | 1.09 |
| 3D2-G8L2 | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D2-G6L4 | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D2-G4L6 | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D2-G2L8 | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D2-L | 8.89 | 20 | 1.59 | 0.63 | 0.63 | / | 1.09 |
| 3D24M1-G6L4 | 8.89 | 20 | 1.62 | 0.55 | 0.65 | 0.65 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Wang, Q.; Ye, J.; He, L.; Guo, L.; Li, X.; Qiu, T.; Tuo, X. The Multi-Step Chain Extension for Waterborne Polyurethane Binder of Para-Aramid Fabrics. Molecules 2022, 27, 7588. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217588
Ma G, Wang Q, Ye J, He L, Guo L, Li X, Qiu T, Tuo X. The Multi-Step Chain Extension for Waterborne Polyurethane Binder of Para-Aramid Fabrics. Molecules. 2022; 27(21):7588. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217588
Chicago/Turabian StyleMa, Ge, Qianshu Wang, Jun Ye, Lifan He, Longhai Guo, Xiaoyu Li, Teng Qiu, and Xinlin Tuo. 2022. "The Multi-Step Chain Extension for Waterborne Polyurethane Binder of Para-Aramid Fabrics" Molecules 27, no. 21: 7588. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27217588