Synthesis and Characterization of Poly(lactic acid) Composites with Organosolv Lignin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Lignin
2.2. Morphology of PLA/Lignin Composites
2.3. FTIR Spectroscopy
2.4. Thermal Properties and Crystallinity
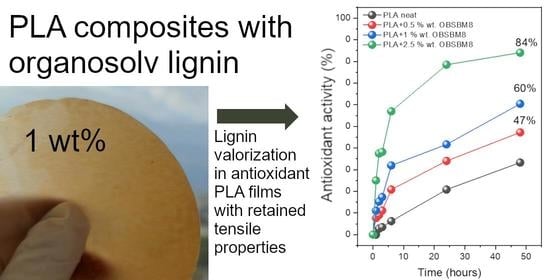
2.5. Antioxidant Activity
2.6. Tensile Properties
3. Materials and Methods
3.1. Materials
3.2. Isolation and Characterization of Lignin
3.3. Preparation of PLA-Lignin Composites
3.3.1. Masterbatch Production via Solution Casting
3.3.2. Fabrication of PLA/Lignin Nanocomposites via Melt Mixing
3.4. Characterization
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Colour Measurements
3.4.3. Fourier-Transformed Infra-Red Spectroscopy (FTIR)
3.4.4. Differential Scanning Calorimetry (DSC)
3.4.5. X-ray Diffraction (XRD)
3.4.6. Antioxidant Activity
3.4.7. Tensile Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- John, M.J.; Lefatle, M.C.; Sithole, B. Lignin Fractionation and Conversion to Bio-Based Functional Products. Sustain. Chem. Pharm. 2022, 25, 100594. [Google Scholar] [CrossRef]
- Yoo, C.G.; Ragauskas, A.J. Lignin Utilization Strategies: From Processing to Applications; American Chemical Society: Washington, DC, USA, 2021; Volume 1377, ISBN 9780841298460. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Kun, D.; Pukánszky, B. Polymer/Lignin Blends: Interactions, Properties, Applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional Lignin-Based Nanocomposites and Nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef] [PubMed]
- Pappa, C.; Feghali, E.; Vanbroekhoven, K.; Triantafyllidis, K.S. Recent Advances in Epoxy Resins and Composites Derived from Lignin and Related Bio-Oils. Curr. Opin. Green Sustain. Chem. 2022, 38, 100687. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of Processing Conditions and Lignin Content on Thermal, Mechanical and Degradative Behavior of Lignin Nanoparticles/Polylactic (Acid) Bionanocomposites Prepared by Melt Extrusion and Solvent Casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Wang, J.; He, Y.; Xie, H.; Zheng, Q. The Influence of Compatibility on the Structure and Properties of PLA/Lignin Biocomposites by Chemical Modification. Polymers 2020, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Kim, J.H.; Lee, S.Y.; Jang, S.K.; Kwak, H.W.; Kim, H.; Choi, I.G. Thermoplasticity Reinforcement of Ethanol Organosolv Lignin to Improve Compatibility in PLA-Based Ligno-Bioplastics: Focusing on the Structural Characteristics of Lignin. Int. J. Biol. Macromol. 2022, 209, 1638–1647. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2020, 26, 126. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.J.; Tawiah, B.; Zhang, Y.; Wang, L.L.; Zhu, S.E.; Chen, T.B.Y.; Yuen, A.C.Y.; Yu, B.; Liu, Y.F.; et al. Synthesis of anhydrous manganese hypophosphite microtubes for simultaneous flame retardant and mechanical enhancement on poly(lactic acid). Compos. Sci. Technol. 2018, 164, 44–50. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.; Hamad, W.Y. Controlling Lignin Particle Size for Polymer Blend Applications. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Cresnar, K.P.; Zamboulis, A.; Bikiaris, D.N.; Aulova, A.; Zemljic, L.F. Kraft Lignin/Tannin as a Potential Accelerator of Antioxidant and Antibacterial Properties in an Active Thermoplastic Polyester-Based Multifunctional Material. Polymers 2022, 14, 1532. [Google Scholar] [CrossRef] [PubMed]
- Črešnar, K.P.; Klonos, P.A.; Zamboulis, A.; Terzopoulou, Z.; Xanthopoulou, E.; Papadopoulos, L.; Kyritsis, A.; Kuzmič, K.; Zemljič, L.F.; Bikiaris, D.N. Structure-Properties Relationships in Renewable Composites Based on Polylactide Filled with Tannin and Kraft Lignin—Crystallization and Molecular Mobility. Thermochim. Acta 2021, 703, 178998. [Google Scholar] [CrossRef]
- Ainali, N.M.; Tarani, E.; Zamboulis, A.; Črešnar, K.P.; Zemljič, L.F.; Chrissafis, K.; Lambropoulou, D.A.; Bikiaris, D.N. Thermal Stability and Decomposition Mechanism of Pla Nanocomposites with Kraft Lignin and Tannin. Polymers 2021, 13, 2818. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent Advances in Organosolv Fractionation: Towards Biomass Fractionation Technology of the Future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Margellou, A.G.; Lazaridis, P.A.; Charisteidis, I.D.; Nitsos, C.K.; Pappa, C.P.; Fotopoulos, A.P.; Van den Bosch, S.; Sels, B.F.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Beech Wood Lignin Isolated by Different Biomass (Pre)Treatment Processes: Organosolv, Hydrothermal and Enzymatic Hydrolysis. Appl. Catal. A Gen. 2021, 623, 118298. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of Technical Lignins for Uses in Biofuels and Biomaterials: Structure-Related Properties, Proximate Analysis and Chemical Modification. Ind. Crops Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Mu, C.; Xue, L.; Zhu, J.; Jiang, M.; Zhou, Z. Mechanical and Thermal Properties of Toughened Poly(L-Lactic) Acid and Lignin Blends. BioResources 2014, 9, 5557–5566. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Cai, C.; Wei, Z.; Wang, P.; Deng, L.; Wang, Y.; Fu, Y. Biobased “Rigid-to-Stretchable” Conversion for Strong and Tough Poly(Lactic Acid) with UV-Protective Property. J. Mater. Process. Technol. 2021, 292, 117052. [Google Scholar] [CrossRef]
- Kim, Y.; Suhr, J.; Seo, H.W.; Sun, H.; Kim, S.; Park, I.K.; Kim, S.H.; Lee, Y.; Kim, K.J.; Nam, J.D. All Biomass and UV Protective Composite Composed of Compatibilized Lignin and Poly (Lactic-Acid). Sci. Rep. 2017, 7, 43596. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Effect of Lignin Nanoparticles and Masterbatch Procedures on the Final Properties of Glycidyl Methacrylate-g-Poly (Lactic Acid) Films before and after Accelerated UV Weathering. Ind. Crops Prod. 2015, 77, 833–844. [Google Scholar] [CrossRef]
- Sammons, R.J.; Harper, D.P.; Labbé, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of Organosolv Lignins Using Thermal and FT-IR Spectroscopic Analysis. BioResources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical Properties of PLA Lignin Blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Pawale, S.; Kalia, K.; Alshammari, S.; Cronin, D.; Zhang, X.; Ameli, A. Deep Eutectic Solvent-Extracted Lignin as an Efficient Additive for Entirely Biobased Polylactic Acid Composites. ACS Appl. Polym. Mater. 2022, 4, 5861–5871. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the Thermal, Dynamic Mechanical and Morphological Properties of PLA-Lignin & PLA-Tannin Particulate Green Composites. Compos. Part B Eng. 2015, 82, 92–99. [Google Scholar] [CrossRef]
- Yetiş, F.; Liu, X.; Sampson, W.W.; Gong, R.H. Acetylation of Lignin Containing Microfibrillated Cellulose and Its Reinforcing Effect for Polylactic Acid. Eur. Polym. J. 2020, 134, 109803. [Google Scholar] [CrossRef]
- Silva, T.F.D.; Menezes, F.; Montagna, L.S.; Lemes, A.P.; Passador, F.R. Effect of Lignin as Accelerator of the Biodegradation Process of Poly(Lactic Acid)/Lignin Composites. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 251, 114441. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA-Lignin Bioplastics Properties before and after Accelerated Weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Tumu, V.R.; Ray Chowdhury, S.; Ramana, R.R. A Green Physical Approach to Compatibilize a Bio-Based Poly (Lactic Acid)/Lignin Blend for Better Mechanical, Thermal and Degradation Properties. Int. J. Biol. Macromol. 2019, 121, 588–600. [Google Scholar] [CrossRef]
- Boarino, A.; Schreier, A.; Leterrier, Y.; Klok, H.A. Uniformly Dispersed Poly(Lactic Acid)-Grafted Lignin Nanoparticles Enhance Antioxidant Activity and UV-Barrier Properties of Poly(Lactic Acid) Packaging Films. ACS Appl. Polym. Mater. 2022, 4, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of Cellulose and Lignin on Disintegration, Antimicrobial and Antioxidant Properties of PLA Active Films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft Lignin as Filler in PLA to Improve Ductility and Thermal Properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Guo, X.; Junna, X.; Wolcott, M.P.; Zhang, J. Mechanochemical Oleation of Lignin through Ball Milling and Properties of Its Blends with PLA. ChemistrySelect 2016, 1, 3449–3454. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal Crystallization Kinetics and Melting Behaviour of Poly(Ethylene Terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]









| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) a | Xc (%) b |
|---|---|---|---|---|---|
| PLA neat | 62.5 | 121.1 | 152.3, 157.1 | 10.3 | 35.0 |
| PLA + 0.5% wt OBS | 59.3 | 122.7 | 150.9, 155.6 | 9.5 | 35.3 |
| PLA + 1% wt OBS | 59.3 | 126.4 | 151.1 | 8.1 | 27.9 |
| PLA + 2.5% wt OBS | 60.4 | 131.7 | 152.8 | 6.5 | 24.5 |
| PLA + 0.5% wt OBSBM8 | 60.4 | 118.9 | 150.8, 155.6 | 9.8 | 26.5 |
| PLA + 1% wt OBSBM8 | 59.9 | 122.9 | 150.8 | 5.6 | 28.4 |
| PLA + 2.5% wt OBSBM8 | 60.6 | 123.7 | 151.5 | 5.8 | 24.4 |
| Sample | Masterbatch | Final Nanocomposite | |
|---|---|---|---|
| PLA (g) | Lignin (g) | ||
| PLA neat | - | - | 10 g PLA |
| PLA + 0.5% wt OBS | 2 | 0.05 | Masterbatch + 7.95 g PLA |
| PLA + 1% wt OBS | 2 | 0.1 | Masterbatch + 7.9 g PLA |
| PLA + 2.5% wt OBS | 2 | 0.25 | Masterbatch + 7.75 g PLA |
| PLA + 0.5% wt OBSBM8 | 2 | 0.05 | Masterbatch + 7.95 g PLA |
| PLA + 1% wt OBSBM8 | 2 | 0.1 | Masterbatch + 7.9 g PLA |
| PLA + 2.5% wt OBSBM8 | 2 | 0.25 | Masterbatch + 7.75 g PLA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzopoulou, Z.; Xanthopoulou, E.; Pardalis, N.; Pappa, C.P.; Torofias, S.; Triantafyllidis, K.S.; Bikiaris, D.N. Synthesis and Characterization of Poly(lactic acid) Composites with Organosolv Lignin. Molecules 2022, 27, 8143. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27238143
Terzopoulou Z, Xanthopoulou E, Pardalis N, Pappa CP, Torofias S, Triantafyllidis KS, Bikiaris DN. Synthesis and Characterization of Poly(lactic acid) Composites with Organosolv Lignin. Molecules. 2022; 27(23):8143. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27238143
Chicago/Turabian StyleTerzopoulou, Zoi, Eleftheria Xanthopoulou, Nikolaos Pardalis, Christina P. Pappa, Stylianos Torofias, Konstantinos S. Triantafyllidis, and Dimitrios N. Bikiaris. 2022. "Synthesis and Characterization of Poly(lactic acid) Composites with Organosolv Lignin" Molecules 27, no. 23: 8143. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27238143