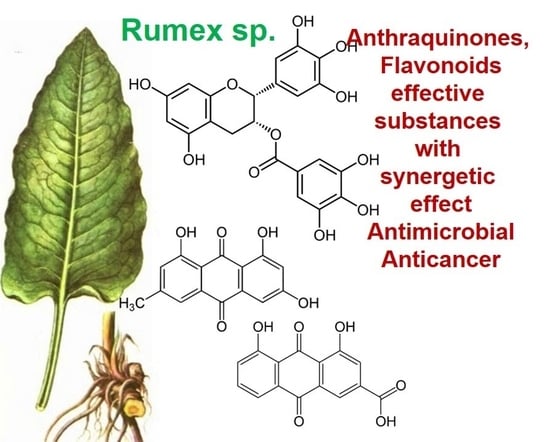
Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species
Abstract
:1. Introduction
1.1. Rumex Confertus Chemical Composition and Prospects for Use
1.2. Rumex Confertus Anticancer Properties
1.3. Rumex Exctracts Anti-Inflammatory and Antioxidant Properties
1.4. Rumex Compounds with Antibacterial, Antimicrobial, and Antiviral Properties
1.5. Antidiabetic Activity of the Plant’s Extract Rumex
1.6. Metabolism of Plant Derivatives In Vivo
2. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Prakash Mishra, A.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bineshian, F.; Bakhshandeh, N.; Freidounian, M.; Nazari, H. Anti-Candida and antioxidant activities of hydroalcohlic extract of Rumex obtusifolius leaves. Pak. J. Pharm. Sci. 2019, 32, 919–926. [Google Scholar] [PubMed]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of Rumex japonicus HOUTT. Aerial parts. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejek, J.; Patykowski, J. Effect of Environmental Factors on Germination and Emergence of Invasive Rumex confertus in Central Europe. Sci. World J. 2015, 2015, 170176. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Smolarz, H.; Wegiera, M.; Wianowska, D.; Dawidowicz, A. Anthracene derivatlves in some species of Rumex L genus. Acta Soc. Bot. Pol. 2007, 76, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Pharmacopeia Russian Federation2.5.0052.15 Roots of Rúmex Confértus. Available online: https://pharmacopoeia.ru/fs-2-5-0052-15-shhavelya-konskogo-korni (accessed on 8 February 2022). (In Russian).
- Xi, N.; Liu, H.; Xu, S.; Yan, X. Qualitative and quantitative analyses of aloe-emodin, rhein, and emodin in qi yin granules by high-performance thin-layer chromatography. JPC—J. Planar Chromatogr.—Mod. TLC 2020, 33, 579–585. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zaitseva, N.V.; Avdeeva, E.V.; Daeva, E.D.; Kadentsev, V.I. Anthraquinones and naphthalene derivatives of Rumex confertus. Chem. Nat. Compd. 2013, 49, 135–137. [Google Scholar] [CrossRef]
- Litvinenko, Y.A.; Muzychkina, R.A. Phytochemical Investigation of Biologically Active Substances in Certain Kazakhstan Rumex Species. 1. Chem. Nat. Compd. 2003, 39, 446–449. [Google Scholar] [CrossRef]
- Guerra, L.; Pereira, C.; Andrade, P.B.; Rodrigues, M.A.; Ferreres, F.; De Pinho, P.G.; Seabra, R.M.; Valentão, P. Targeted metabolite analysis and antioxidant potential of Rumex induratus. J. Agric. Food Chem. 2008, 56, 8184–8194. [Google Scholar] [CrossRef]
- Crews, C.; Clarke, D. Natural Toxicants: Naturally Occurring Toxins of Plant Origin. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 261–268. ISBN 978-0-12-378613-5. [Google Scholar]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 8 February 2022).
- Chen, M.; Cai, F.; Zha, D.; Wang, X.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.-C. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. Oncotarget 2017, 8, 26941–26958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodor, N.; Buchwald, P. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Adv. Drug Deliv. Rev. 1999, 36, 229–254. [Google Scholar] [CrossRef]
- Henderson, B.W.; Bellnier, D.A.; Greco, W.R.; Sharma, A.; Pandey, R.K.; Vaughan, L.A.; Weishaupt, K.R.; Dougherty, T.J. An in vivo quantitative structure-activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res. 1997, 57, 4000–4007. [Google Scholar] [PubMed]
- Yang, B.; Chen, F.; Hua, Y.; Huang, S.-S.; Lin, S.; Wen, L.; Jiang, Y. Prooxidant activities of quercetin, p-courmaric acid and their derivatives analysed by quantitative structure–activity relationship. Food Chem. 2012, 131, 508–512. [Google Scholar] [CrossRef]
- PubChem Rhein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10168 (accessed on 17 December 2021).
- Bachmann, M.; Schlatter, C. Metabolism of [14C]emodin in the rat. Xenobiotica Fate Foreign Compd. Biol. Syst. 1981, 11, 217–225. [Google Scholar] [CrossRef]
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Vecchia, F.D.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M.; et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804. [Google Scholar]
- Zu, C.; Zhang, M.; Xue, H.; Cai, X.; Zhao, L.; He, A.; Qin, G.; Yang, C.; Zheng, X. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol. Lett. 2015, 10, 2919–2924. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-G.; Chen, G.-W.; Li, T.-M.; Chouh, S.-T.; Tan, T.-W.; Chung, J.-G. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. J. Urol. 2006, 175, 343–347. [Google Scholar] [CrossRef]
- Fernand, V.E.; Losso, J.N.; Truax, R.E.; Villar, E.E.; Bwambok, D.K.; Fakayode, S.O.; Lowry, M.; Warner, I.M. Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro. Chem. Biol. Interact. 2011, 192, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Neagoe, I.B. The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.-C.; Yang, J.-S.; Huang, A.-C.; Hsia, T.-C.; Chou, S.-T.; Kuo, C.-L.; Lu, H.-F.; Lee, T.-H.; Wood, W.G.; Chung, J.-G. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol. Nutr. Food Res. 2010, 54, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, N.-N.; Li, H.-Y.; Jiang, M.; Gao, J.; Bai, G. Active ingredients in rhubarb with anti-proliferative effects on scar fibroblasts. Yao Xue Xue Bao 2012, 47, 1618–1622. [Google Scholar]
- Kuo, P.-L.; Lin, T.-C.; Lin, C.-C. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002, 71, 1879–1892. [Google Scholar] [CrossRef]
- Lee, H.Z.; Hsu, S.L.; Liu, M.C.; Wu, C.H. Effects and mechanisms of aloe-emodin on cell death in human lung squamous cell carcinoma. Eur. J. Pharmacol. 2001, 431, 287–295. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, K.-Y.; Chang, C.-C.; Fang, C.-L.; Lin, C.-P. Aloe-emodin-induced apoptosis in human gastric carcinoma cells. Food Chem. Toxicol. 2007, 45, 2296–2303. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, X.; Wang, L.; Li, X.; Xie, T.; Zhao, J.; Yan, J.; Wang, L.; Ye, H.; Jiao, S.; et al. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics 2020, 10, 6839–6853. [Google Scholar] [CrossRef]
- Antonyan, A.; Sharoyan, S.; Harutyunyan, H.; Barboni, L.; Lupidi, G.; Mardanyan, S. Protection of hippocampal and islet beta cells in vitro by emodin from leaves of Rumex confertus. Int. J. Pharmacogn. Phytochem. Res. 2016, 3, 437–444. [Google Scholar] [CrossRef]
- Chun-Guang, W.; Jun-Qing, Y.; Bei-Zhong, L.; Dan-Ting, J.; Chong, W.; Liang, Z.; Dan, Z.; Yan, W. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur. J. Pharmacol. 2010, 627, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Peterse, J.L.; van ’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xu, X.; Zhang, Q.; Fu, G.; Mo, Z.; Wang, G.S.; Kishi, S.; Yang, X.-L. tRNA synthetase counteracts c-Myc to develop functional vasculature. eLife 2014, 3, e02349. [Google Scholar] [CrossRef]
- Bai, J.; Wu, J.; Tang, R.; Sun, C.; Ji, J.; Yin, Z.; Ma, G.; Yang, W. Emodin, a natural anthraquinone, suppresses liver cancer in vitro and in vivo by regulating VEGFR2 and miR-34a. Investig. New Drugs 2020, 38, 229–245. [Google Scholar] [CrossRef]
- Gu, J.; Cui, C.-F.; Yang, L.; Wang, L.; Jiang, X.-H. Emodin Inhibits Colon Cancer Cell Invasion and Migration by Suppressing Epithelial-Mesenchymal Transition via the Wnt/β-Catenin Pathway. Oncol. Res. 2019, 27, 193–202. [Google Scholar] [CrossRef]
- Dai, G.; Ding, K.; Cao, Q.; Xu, T.; He, F.; Liu, S.; Ju, W. Emodin suppresses growth and invasion of colorectal cancer cells by inhibiting VEGFR2. Eur. J. Pharmacol. 2019, 859, 172525. [Google Scholar] [CrossRef]
- National Toxicology Program. Photocarcinogenesis Study of Aloe Vera [CAS NO. 481-72-1(Aloe-emodin)] in SKH-1 Mice (Simulated Solar Light and Topical Application Study). Natl. Toxicol. Program. Tech. Rep. Ser. 2010. Available online: https://pubmed.ncbi.nlm.nih.gov/21031007/ (accessed on 8 February 2022).
- Jiang, D.; Ding, S.; Mao, Z.; You, L.; Ruan, Y. Integrated analysis of potential pathways by which aloe-emodin induces the apoptosis of colon cancer cells. Cancer Cell Int. 2021, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, J.; Yu, K.; Shu, Y.; He, W.; Chu, H.; Zhang, B.; Ge, C. Aloe-emodin induces apoptosis in human oral squamous cell carcinoma SCC15 cells. BMC Complement. Altern. Med. 2018, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, W.-W.; He, M.; Wei, D.; Long, Z.-J.; Tao, Y.-M. Aloe emodin inhibits telomerase activity in breast cancer cells: Transcriptional and enzymological mechanism. Pharmacol. Rep. 2020, 72, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Dong, W. Aloe-Emodin Induces Endoplasmic Reticulum Stress-Dependent Apoptosis in Colorectal Cancer Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6331–6339. [Google Scholar] [CrossRef]
- Shen, F.; Ge, C.; Yuan, P. Aloe-emodin induces autophagy and apoptotic cell death in non-small cell lung cancer cells via Akt/mTOR and MAPK signaling. Eur. J. Pharmacol. 2020, 886, 173550. [Google Scholar] [CrossRef]
- Shi, P.; Huang, Z.; Chen, G. Rhein induces apoptosis and cell cycle arrest in human hepatocellular carcinoma BEL-7402 cells. Am. J. Chin. Med. 2008, 36, 805–813. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Shao, T.; Song, P.; Kong, Q.; Hua, H.; Luo, T.; Jiang, Y. The natural agent rhein induces β-catenin degradation and tumour growth arrest. J. Cell. Mol. Med. 2018, 22, 589–599. [Google Scholar] [CrossRef]
- You, L.; Dong, X.; Yin, X.; Yang, C.; Leng, X.; Wang, W.; Ni, J. Rhein Induces Cell Death in HepaRG Cells through Cell Cycle Arrest and Apoptotic Pathway. Int. J. Mol. Sci. 2018, 19, 1060. [Google Scholar] [CrossRef] [Green Version]
- Azzam, M.M.; Qaid, M.M.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Albaadani, H.H.; Alhidary, I.A. Rumex nervosus leaves meal improves body weight gain, duodenal morphology, serum thyroid hormones, and cecal microflora of broiler chickens during the starter period. Poult. Sci. 2020, 99, 5572–5581. [Google Scholar] [CrossRef]
- Demirezer, L.O.; Kuruüzüm-Uz, A.; Bergere, I.; Schiewe, H.J.; Zeeck, A. The structures of antioxidant and cytotoxic agents from natural source: Anthraquinones and tannins from roots of Rumex patientia. Phytochemistry 2001, 58, 1213–1217. [Google Scholar] [CrossRef]
- El-Hawary, S.A.; Sokkar, N.M.; Ali, Z.Y.; Yehia, M.M. A profile of bioactive compounds of Rumex vesicarius L. J. Food Sci. 2011, 76, C1195–C1202. [Google Scholar] [CrossRef]
- Shafiq, N.; Noreen, S.; Rafiq, N.; Ali, B.; Parveen, S.; Mahmood, A.; Sajid, A.; Akhtar, N.; Bilal, M. Isolation of bioactive compounds from Rumex hastatus extract and their biological evaluation and docking study as potential anti-oxidant and anti-urease agents. J. Food Biochem. 2020, 44, e13320. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, K.; Mao, Y.-H.; Qu, H.; Yao, B.; Zhong, W.-W.; Ma, B.; Wang, Z.-Y. Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int. J. Oncol. 2017, 51, 1089–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.-C.; Hsieh, C.-H.; Hsiao, M.-W.; Lin, W.-C.; Hung, Y.-C.; Ye, J.-C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan. J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.K.; Khan, M.A.; Singh, S.K. Constitutive Inflammatory Cytokine Storm: A Major Threat to Human Health. J. Interferon Cytokine Res. 2020, 40, 19–23. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, H.; Yang, D.; He, F.; Yuan, Y.; Guo, J.; Hu, J.; Yu, J.; Yan, X.; Wang, S.; et al. Aloe-emodin attenuates myocardial infarction and apoptosis via up-regulating miR-133 expression. Pharmacol. Res. 2019, 146, 104315. [Google Scholar] [CrossRef]
- Wu, Y.; Tu, X.; Lin, G.; Xia, H.; Huang, H.; Wan, J.; Cheng, Z.; Liu, M.; Chen, G.; Zhang, H.; et al. Emodin-mediated protection from acute myocardial infarction via inhibition of inflammation and apoptosis in local ischemic myocardium. Life Sci. 2007, 81, 1332–1338. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.; Pang, X.; Chen, A.; Wang, Y. Molecular Mechanisms of Action of Emodin: As an Anti-Cardiovascular Disease Drug. Front. Pharmacol. 2020, 11, 559607. [Google Scholar] [CrossRef]
- Wei, G.; Wu, Y.; Gao, Q.; Zhou, C.; Wang, K.; Shen, C.; Wang, G.; Wang, K.; Sun, X.; Li, X. Effect of Emodin on Preventing Postoperative Intra-Abdominal Adhesion Formation. Oxid. Med. Cell. Longev. 2017, 2017, 1740317. [Google Scholar] [CrossRef] [Green Version]
- Kitano, A.; Saika, S.; Yamanaka, O.; Ikeda, K.; Okada, Y.; Shirai, K.; Reinach, P.S. Emodin suppression of ocular surface inflammatory reaction. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Emodin in Rheum undulatum inhibits oxidative stress in the liver via AMPK with Hippo/Yap signalling pathway. Pharm. Biol. 2020, 58, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Huang, R.-S.; Yu, X.-X.; Ye, Q.; Pan, L.-L.; Shao, G.-J.; Pan, J. Emodin protects against oxidative stress and apoptosis in HK-2 renal tubular epithelial cells after hypoxia/reoxygenation. Exp. Ther. Med. 2017, 14, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Waly, M.I.; Ali, B.H.; Al-Lawati, I.; Nemmar, A. Protective effects of emodin against cisplatin-induced oxidative stress in cultured human kidney (HEK 293) cells. J. Appl. Toxicol. 2013, 33, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-J.; Ma, Y.-H.; Miao, L.; Wang, Y.; Wang, H.-Z.; Xing, Y.-Y.; Xi, T.; Lu, Y.-Y. Emodin-provoked oxidative stress induces apoptosis in human colon cancer HCT116 cells through a p53-mitochondrial apoptotic pathway. Asian Pac. J. Cancer Prev. 2014, 15, 5201–5205. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-Y.; Kwon, H.-J.; Sung, M.-K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef]
- Li, L.; Song, X.; Yin, Z.; Jia, R.; Li, Z.; Zhou, X.; Zou, Y.; Li, L.; Yin, L.; Yue, G.; et al. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016, 186–187, 139–145. [Google Scholar] [CrossRef]
- Orbán-Gyapai, O.; Liktor-Busa, E.; Kúsz, N.; Stefkó, D.; Urbán, E.; Hohmann, J.; Vasas, A. Antibacterial screening of Rumex species native to the Carpathian Basin and bioactivity-guided isolation of compounds from Rumex aquaticus. Fitoterapia 2017, 118, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginovyan, M.; Ayvazyan, A.; Nikoyan, A.; Tumanyan, L.; Trchounian, A. Phytochemical Screening and Detection of Antibacterial Components from Crude Extracts of Some Armenian Herbs Using TLC-Bioautographic Technique. Curr. Microbiol. 2020, 77, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, L.; Gayen, J.R.; Sinha, S.; Pal, S.; Pal, M.; Saha, B.P. Antibacterial efficacy of Rumex nepalensis Spreng. roots. Phytother. Res. 2003, 17, 558–559. [Google Scholar] [CrossRef]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, K.M.; Mandal, A.K.; Rajesh, J.; Natarajan, S. Antioxidant and Antimicrobial Activity of Individual Catechin Molecules: A Comparative Study between Gallated and Epimerized Catechin Molecules. Res. J. Biotechnol. 2012, 7, 5–8. [Google Scholar]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.-D.-D. Antibacterial, antifungal and antioxidant activities of whole plant chemical constituents of Rumex abyssinicus. BMC Complement. Med. Ther. 2021, 21, 164. [Google Scholar] [CrossRef]
- Kim, G.; Xu, Y.J.; Farha, A.K.; Sui, Z.Q.; Corke, H. Bactericidal and antibiofilm properties of Rumex japonicus Houtt. on multidrug-resistant Staphylococcus aureus isolated from milk. J. Dairy Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Noshad, M.; Alizadeh Behbahani, B. Investigation of chemical properties and antimicrobial activity of Rumex alveollatus methanolic extract on Enterobacter aerogenes, Staphylococcus aureus, Salmonella typhi, and Streptococcus pyogenes: An “in vitro” study. Food Sci. Technol. 2021, 18, 109–117. [Google Scholar] [CrossRef]
- Xu, J.-S.; Cui, Y.; Liao, X.-M.; Tan, X.-B.; Cao, X. Effect of emodin on the cariogenic properties of Streptococcus mutans and the development of caries in rats. Exp. Ther. Med. 2014, 8, 1308–1312. [Google Scholar] [CrossRef] [Green Version]
- Huy, T.X.N.; Reyes, A.W.B.; Hop, H.T.; Arayan, L.T.; Son, V.H.; Min, W.; Lee, H.J.; Kim, S. Emodin Successfully Inhibited Invasion of Brucella abortus Via Modulting Adherence, Microtubule Dynamics and ERK Signaling Pathway in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2018, 28, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Bhardwaj, R.; Sharma, P.; Yadav, A.; Singh, B. Antimicrobial activity of sennosides from Cassia pumila Lamk. J. Med. Plants Res. 2012, 6, 3591–3595. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E.; Kozukue, N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 2006, 69, 354–361. [Google Scholar] [CrossRef]
- Gradisar, H.; Pristovsek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Coopoosamy, R.M.; Magwa, M.L. Antibacterial activity of chrysophanol isolated from Aloe excelsa (Berger). Afr. J. Biotechnol. 2006, 5. [Google Scholar] [CrossRef]
- Rath, J.P.; Raval, M. Structure based screening of ligands against dTDP-6-deoxy-D-xylo-4-hexulose 3, 5-epimerase (RmlC): Phytochemical as drug candidate for Mycobacterium tuberculosis. Pharm. Biol. Eval. 2017, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Kawai, K.; Beppu, H.; Shimpo, K.; Chihara, T.; Yamamoto, N.; Nagatsu, T.; Ueda, H.; Yamada, Y. In vivo effects of Aloe arborescens Miller var. natalensis Berger (Kidachi aloe) on experimental tinea pedis in guinea-pig feet. Phytother. Res. 1998, 12, 178–182. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, F.; Qiu, Y.; Ye, X.; Hanson, P.; Shen, H.; Yang, D. Emodin inhibits coxsackievirus B3 replication via multiple signalling cascades leading to suppression of translation. Biochem. J. 2016, 473, 473–485. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wu, C.-F.; Hsiao, N.-W.; Chang, C.-Y.; Li, S.-W.; Wan, L.; Lin, Y.-J.; Lin, W.-Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef]
- Chang, S.-J.; Huang, S.-H.; Lin, Y.-J.; Tsou, Y.-Y.; Lin, C.-W. Antiviral activity of Rheum palmatum methanol extract and chrysophanol against Japanese encephalitis virus. Arch. Pharm. Res. 2014, 37, 1117–1123. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, T.-C.; Lai, C.-C.; Huang, S.-H.; Liao, J.-M.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef]
- Bhimaneni, S.; Kumar, A. Abscisic acid and aloe-emodin against NS2B-NS3A protease of Japanese encephalitis virus. Environ. Sci. Pollut. Res. 2022, 29, 8759–8766. [Google Scholar] [CrossRef]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [CrossRef]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.-H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermark, P.; Andersson, A.; Westermark, G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011, 91, 795–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, C.J.; Burdick, D.; Walencewicz, A.J.; Glabe, C.G.; Cotman, C.W. Neurodegeneration induced by beta-amyloid peptides in vitro: The role of peptide assembly state. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 1676–1687. [Google Scholar] [CrossRef]
- Alghamdi, A.; Vyshemirsky, V.; Birch, D.J.S.; Rolinski, O.J. Detecting beta-amyloid aggregation from time-resolved emission spectra. Methods Appl. Fluoresc. 2018, 6, 024002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jin, H.; Sun, Q.-R.; Xu, J.-H.; Hu, H.-T. Neuroprotective effects of emodin in rat cortical neurons against beta-amyloid-induced neurotoxicity. Brain Res. 2010, 1347, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Muthusamy, V.S.; Sujatha, S.; Sangeetha, K.N.; Bharathi Raja, R.; Sudhagar, S.; Poornima Devi, N.; Lakshmi, B.S. Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 2010, 584, 3170–3178. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Ullah, F.; Ayaz, M.; Ahmad, A.; Sadiq, A.; Mohani, S.N.-U.-H. Nutritional and medicinal aspects of Rumex hastatus D. Don along with in vitro anti-diabetic activity. Int. J. Food Prop. 2019, 22, 1733–1748. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Ding, W.; Liu, Y. Anti-diabetic effects of emodin involved in the activation of PPARgamma on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia 2010, 81, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, C.; Vilella, C.A.; Vieira, K.P.; Souza, G.H.M.F.; Hyslop, S.; Zollner, R.d.L. Diacerhein downregulate proinflammatory cytokines expression and decrease the autoimmune diabetes frequency in nonobese diabetic (NOD) mice. Int. Immunopharmacol. 2008, 8, 782–791. [Google Scholar] [CrossRef]
- Jia, Z.H.; Liu, Z.H.; Zheng, J.M.; Zeng, C.H.; Li, L.S. Combined therapy of rhein and benazepril on the treatment of diabetic nephropathy in db/db mice. Exp. Clin. Endocrinol. Diabetes 2007, 115, 571–576. [Google Scholar] [CrossRef]
- Choi, S.B.; Ko, B.S.; Park, S.K.; Jang, J.S.; Park, S. Insulin sensitizing and alpha-glucoamylase inhibitory action of sennosides, rheins and rhaponticin in Rhei Rhizoma. Life Sci. 2006, 78, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Yonezawa, T.; Son, M.J.; Woo, J.T.; Ohba, S.; Chung, U.-I.; Yagasaki, K. Antidiabetic effect of nepodin, a component of Rumex roots, and its modes of action in vitro and in vivo. BioFactors 2014, 40, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Kumar, V.; Singh, V.; Bairwa, K.; Sobhia, M.E.; Jachak, S.M. Synthesis, biological evaluation, molecular docking and theoretical evaluation of ADMET properties of nepodin and chrysophanol derivatives as potential cyclooxygenase (COX-1, COX-2) inhibitors. Eur. J. Med. Chem. 2014, 80, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Yang, F. Barbaloin Treatment Contributes to the Rebalance of Glucose and Lipid Homeostasis of Gestational Diabetes Mellitus Mice. Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820984910. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Hu, X.-Q.; Ouyang, J.; Dai, B.; Xu, Y. The effect of astragalin on the VEGF production of cultured Müller cells under high glucose conditions. Biomed. Mater. Eng. 2012, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, J.M.; LaBarbera, D.V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.-H.; Huang, C.-Y.; Chen, M.-C.; Lee, Y.-T.; Yue, C.-H.; Wang, H.-Y.; Lin, H. Emodin and Aloe-Emodin Suppress Breast Cancer Cell Proliferation through ERα Inhibition. Evid. Based Complement. Alternat. Med. 2013, 2013, e376123. [Google Scholar] [CrossRef] [Green Version]
- Haskell, C. Cerebral Blood Flow, Cerebro-electrical Activity and Behavioural Effects of Epigallocatechin Gallate (EGCG) Administration in Healthy, Young Adults. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT00981292 (accessed on 27 January 2022).
- He, Y.; Huang, J.; Wang, P.; Shen, X.; Li, S.; Yang, L.; Liu, W.; Suksamrarn, A.; Zhang, G.; Wang, F. Emodin potentiates the antiproliferative effect of interferon α/β by activation of JAK/STAT pathway signaling through inhibition of the 26S proteasome. Oncotarget 2015, 7, 4664–4679. [Google Scholar] [CrossRef] [Green Version]
- Medical University of Warsaw. High.-Volume Polyethylene Glycol Solution (PEG) Versus Low-Volume PEG Plus Stimulant Laxative Versus Sennosides for Colon Cleansing Before Colonoscopy: A Randomized, Single Blinded Study. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01531140 (accessed on 27 January 2022).
- Liu, Z.-H. Randomized Clinical Trial of Triptolide Woldifii for Autosomal Dominant Polycystic Kidney Disease (ADPKD). 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT00801268 (accessed on 27 January 2022).
- H. Lee Moffitt Cancer Center and Research Institute. Phase II, Randomized, Double-blind, Multi-centered Study of Polyphenon E in Men With High-grade Prostatic Intraepithelial Neoplasia (HGPIN) or Atypical Small Acinar Proliferation (ASAP). Available online: https://clinicaltrials.gov/ct2/show/NCT00596011 (accessed on 27 January 2022).
- Selbach, S.; Klocke, A.; Peters, U.; Beckert, S.; Watt, R.M.; Tong, R.; Flemmig, T.F.; Hensel, A.; Beikler, T. Microbiological and clinical effects of a Proanthocyanidin-enriched extract from Rumex acetosa in periodontally healthy carriers of Porphyromonas gingivalis. Planta Med. 2021. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Yu, X. Determination of sennosides and degraded products in the process of sennoside metabolism by HPLC. Se Pu Chin. J. Chromatogr. 2004, 22, 48–50. [Google Scholar]
- Mueller, S.O.; Stopper, H.; Dekant, W. Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites. Drug Metab. Dispos. Biol. Fate Chem. 1998, 26, 540–546. [Google Scholar] [PubMed]
- Sun, Y.; Xin, X.; Zhang, K.; Cui, T.; Peng, Y.; Zheng, J. Cytochrome P450 mediated metabolic activation of chrysophanol. Chem. Biol. Interact. 2018, 289, 57–67. [Google Scholar] [CrossRef] [PubMed]



 |  |  | |
| Emodin 6-methyl-1,3,8-trihydroxyanthraquinone Logp 2.7 water solubility less than 1 mg/mL at 66 °F. Solubility at 25 °C (g/100 mL of saturated solution): ether 0.140; chloroform 0.071 [16]; Ld50 mouse intraperitoneal 35 mg/kg [21]. | Aloe-emodin 1,8-dihydroxy-3-(hydroxymethyl) anthraquinone LogP 3.25 [22], water solubility 5.5 × 10−5 mole/L LD50 mouse intraperitoneal 35 mg/kg [23]. | Rhein 4,5-dihydroxy-9,10-dioxo-anthracene-2-carboxylic acid LogP 2.2 water solubility less than 1 mg/mL at 66 °F LD50 mouse oral 5000 mg/kg LD50 mouse intraperitoneal 25 mg/kg [24]. | |
 |  |  | |
| Leucocyanidin (2R,3S,4S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,4,5,7-tetrol Water Solubility 2.22 g/L pKa 8.95 LD50 2110 mg/kg Rat Oral admin [25]. | Chrysophanol or chrysophanic acid (1,8-dihydroxy-3-methylanthracene-9,10-dione) Water Solubility 0.12 g/L logP 4.12 pKa 9.14 [26]. | Physcion (1,8-Dihydroxy-6-methoxy-3-methylanthraquinone) or emodin monomethyl ether Water Solubility 0.087 g/L logP 3.97 pKa 7.89 LD50 mouse intraperitoneal 10 mg/kg [27]. | |
 |  | ||
| Epigallocatechin gallate (EGCG) water solubility 32.77 mg/L at 25 °C LogP 0.639 pKa 7.75 LD50 2170 mg/kg at oral administration [28]. | Epigallocatechin (EGC) water solubility 0.871 mg/mL LogP 0.71 pKa 8.73 LD50 1.87 mol/kg = 857 mg/kg [29]. | ||
 |  | ||
| Barbaloin or Aloin A 1,8-dihydroxy-3-(hydroxymethyl)-10-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-10H-anthracen-9-one water solubility 2.63 g/mL [30] Log P 0.42 pKa 9.51 LD50 cat oral 500 mg/kg LD50 mouse intraperitoneal 200 mg/kg [31]. | Sennoside (-)-(9R*,9′R*)-5,5′-bis(β-D-gluco-pyranosyloxy)-4,4′-dihydroxy-10,10′-dioxo-9,9′,10,10′-tetrahydro-9,9′-bianthracene-2,2′-dicarboxylic acid water solubility 0.753 mg/mL LogP1.2 The LD50 value in rats was 5000 mg/kg [32] pka 3.23 [33]. | ||
| Compound | Effect | Action | Clinical Experiment |
|---|---|---|---|
| Epigallocatechin gallate [112] | neuroprotective and neurorescue effects | modulation of cell survival and cell cycle genes | A randomised, double-blind, placebo-controlled, balanced crossover study the effects of 135 mg and 270 mg pure EGCG in 24 healthy, young adults (18–35) |
| Emodin [113] | antiproliferative effect | inhibits the activity of the 26S proteasome in vitro and in vivo | HEK293A-luciferase-cODC cells were seeded in 96-well plates and treated in the presence of emodin |
| Sennoside [114] | laxative | increasing cyclic 3′,5′-adenosine monophosphate it alter permeability of cell walls in the colon, which regulates the process of active ion secretion | Participants aged 10–18 years were randomly assigned to receive either PEG 60 mL/kg/day or PEG 30 mL/kg/day plus oral bisacodyl 10–15 mg/day or sennosides 2 mg/kg/day for 2 days prior to the colonoscopy |
| Aloe-emodin [115] | Triptolide Woldifii for Autosomal Dominant Polycystic Kidney Disease | MRI calculated kidney volume, eGFR [Time Frame: Every 3–6 months] End-stage kidney disease (ESRD) [Time Frame: every 2 months] | Interventional 300 participants from 15 to 70 years (child, adult, older adult) Randomized administration of Emodin (Frangula emodin, Frangulic acid) at concentration 100 mg/d |
| Epigallocatechin anticancer drug [116] | To determine whether the daily consumption of decaffeinated green tea catechins (Polyphenon E®) for 1 year reduces the rate of progression to prostate cancer (PCa) in men diagnosed with HGPIN or ASAP | Study of Polyphenon E in Men with High-grade Prostatic Intraepithelial Neoplasia | 240 (120 men/arm) men 30 Years to 80 Years (adult, older adult) diagnosed with the prostate condition HGPIN or ASAP with a capsule form of standardized green tea extract called Polyphenon E (200 mg epigallocatechin gallate (EGCG) twice a day) or placebo for a 12-month period and see if it can prevent progression of the prostate condition to prostate cancer. Investigators wanted to see if Polyphenon E reduces lower urinary tract symptoms and if this can be taken safely over one year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041204
Berillo D, Kozhahmetova M, Lebedeva L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules. 2022; 27(4):1204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041204
Chicago/Turabian StyleBerillo, Dmitriy, Marzhan Kozhahmetova, and Lina Lebedeva. 2022. "Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species" Molecules 27, no. 4: 1204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041204