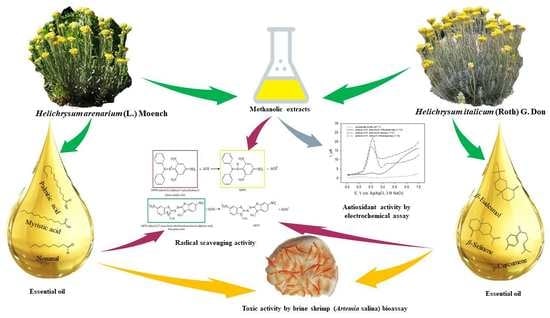
Antioxidant and Toxic Activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don Essential Oils and Extracts
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Essential Oils
2.2. Chemical Composition of Methanolic Extracts
2.3. Total Phenolic Content
2.4. Antioxidant Activity Tests
2.4.1. Spectrophotometric (DPPH● and ABTS●+) Assays
2.4.2. Electrochemical (Cyclic and Square Wave Voltammetry) Assays
2.5. Toxic Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Isolation
4.3. Preparation of Extracts
4.4. GC (Flame-Ionization Detector FID) Analysis
4.5. GC-MS Analysis
4.6. Identification of Individual Components
4.7. HPLC-DAD-MS (TOF) Analysis
4.8. Determination of Total Phenolic Content (TPC)
4.9. Antioxidant Activity
4.9.1. Spectrophotometric Assays
Antioxidant Capacity ABTS●+ Assay
DPPH● Assay
TROLOX Equivalent ABTS●+ and DPPH● Assays
4.9.2. Electrochemical (Cyclic and Square Wave Voltammetry) Analysis
4.10. Toxicity Test
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Galbany-Casals, M.; Sáez, L.; Benedí, C. A taxonomic revision of Helichrysum sect. Stoechadina (Asteraceae, Gnaphalieae). Can. J. Bot. 2006, 84, 1203–1232. [Google Scholar] [CrossRef]
- Galbany-Casals, M.; Blanco-Moreno, J.M.; Garcia-Jacas, N.; Breitwieser, I.; Smissen, R.D. Genetic variation in Mediterranean Helichrysum italicum (Asteraceae; Gnaphalieae): Do disjunction populations of subsp. microphyllum have a common origin? Plant Biol. 2011, 13, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Ninčević, T.; Grdiša, M.; Šatović, Z.; Jug-Dujaković, M. Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity. Ind. Crop. Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of phytochemicals from Helichrysum italicum: An analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry 2017, 138, 9–28. [Google Scholar] [CrossRef]
- Morone-Fortunato, I.; Montemurro, C.; Ruta, C.; Perrini, R.; Sabetta, W.; Blanco, A.; Lorusso, E.; Avato, P. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. italicum from wild Mediterranean germplasm. Ind. Crop. Prod. 2010, 32, 639–649. [Google Scholar] [CrossRef]
- Usaï, M.; Foddai, M.; Bernardini, A.F.; Muselli, A.; Costa, J.; Marchetti, M. Chemical composition and variation of the essential oil of wild sardinian Helichrysum italicum G. Don subsp. microphyllum (Willd.) Nym from vegetative period to post-blooming. J. Essent. Oil Res. 2010, 22, 373–380. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Ivanović, J.; Ristić, M.; Skal, D. Supercritical CO2 extraction of Helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar]
- Mancini, E.; De Martino, L.; Marandino, A.; Scognamiglio, M.R.; De Feo, V. Chemical composition and possible in vitro phytotoxic activity of Helichrysum italicum (Roth) Don ssp. italicum. Molecules 2011, 16, 7725–7735. [Google Scholar] [CrossRef] [Green Version]
- Cristofari, G.; Znini, M.; Majidi, L.; Costa, J.; Hammouti, B.; Paolini, J. Helichrysum italicum subsp. italicum essential oil as environmentally friendly inhibitor on the corrosion of mil steel in hydrochloric acid. Int. J. Electrochem. Sci. 2012, 7, 9024–9041. [Google Scholar]
- Leonardi, M.; Ambryszewska, K.E.; Melai, B.; Flamini, G.; Cioni, P.L.; Parri, F.; Pistelli, L. Essential oil composition of Helichrysum italicum (Roth) G.Don ssp. italicum from Elba island (Tuscany, Italy). Chem. Biodivers. 2013, 10, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Melito, S.; Sias, A.; Petretto, G.L.; Chessa, M.; Pintore, G.; Porceddu, A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE 2013, 8, e79043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimović, S.; Kesić, Z.; Lukić, I.; Milovanović, S.; Ristić, M.; Skala, D. Supercritical fluid extraction of curry flowers, sage leaves, and their mixture. J. Supercrit. Fluids 2013, 84, 1–12. [Google Scholar] [CrossRef]
- Stupar, M.; Ljaljević-Grbić, M.; Džamić, A.; Unković, N.; Ristić, M.; Vukojević, J. Antifungal activity of Helichrysum italicum (Roth) G. Don (Asteraceae) essential oil against fungi isolated from cultural heritage objects. Arch. Biol. Sci. 2014, 66, 1539–1545. [Google Scholar] [CrossRef]
- Zeljković, S.Ć.; Šolić, M.E.; Maksimović, M. Volatiles of Helichrysum italicum (Roth) G. Don from Croatia. Nat. Prod. Res. 2015, 29, 1874–1877. [Google Scholar] [CrossRef]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Božin, B.N. Biochemical characterization of Helichrysum italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar] [CrossRef]
- Costa, P.; Loureiro, J.M.; Teixeira, M.A.; Rodrigues, A.E. Extraction of aromatic volatiles by hydrodistillation and supercritical fluid extraction with CO2 from Helichrysum italicum subsp. picardii growing in Portugal. Ind. Crop. Prod. 2015, 77, 680–683. [Google Scholar] [CrossRef]
- Schipilliti, L.; Bonaccorsi, I.L.; Ragusa, S.; Cotroneo, A.; Dugo, P. Helichrysum italicum (Roth) G. Don fil. subsp. italicum oil analysis by gas chromatography—Carbon isotope ratio mass spectrometry (GC-C-IRMS): A rapid method of genotype differentiation? J. Essent. Oil Res. 2016, 28, 193–201. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition. Ind. Crops Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Bouchaala, M.; Ramdani, M.; Lograda, T.; Chalard, P.; Figueredo, G. Chemical composition, antibacterial activity and chromosome number of Helichrysum italicum from Algeria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1675–1683. [Google Scholar]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, D.H.J.; Maria, A.E.; Mihoub, Z.M. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against gram-positive and gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Beaumont, C.; Stevens, N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Staver, M.M.; Gobin, I.; Ratkaj, I.; Petrovic, M.; Vulinovic, A.; Dinarina-Sablic, M.; Broznic, D. In vitro Antiproliferative and antimicrobial activity of the essential oil from the flowers and leaves of Helichrysum italicum (Roth) G. Don growing in Central Dalmatia (Croatia). J. Essent. Oil-Bear. Plants 2018, 21, 77–91. [Google Scholar] [CrossRef]
- Tzanova, M.; Grozeva, N.; Gerdzhikova, M.; Atanasov, V.; Terzieva, S.; Prodanova, R. Biochemical composition of essential oil of Corsican Helichrysum italicum (Roth) G. Don, introduced and cultivated in South Bulgaria. Bulg. J. Agric. Sci. 2018, 24, 1071–1077. [Google Scholar]
- Odak, I.; Lukic, T.; Talic, S. Impact of storage conditions on alteration of juniper and immortelle essential oils. J. Essent. Oil-Bear. Plants 2018, 21, 614–622. [Google Scholar] [CrossRef]
- Dzamic, A.M.; Mileski, K.S.; Ciric, A.D.; Ristic, M.S.; Sokovic, M.D.; Marin, P.D. Essential oil composition, antioxidant and antimicrobial properties of essential oil and deodorized extracts of Helichrysum italicum (Roth) G. Don. J. Essent. Oil-Bear. Plants 2019, 22, 493–503. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ascrizzi, R. In vitro Anticollagenase and antielastase activities of essential oil of Helichrysum italicum subsp. italicum (Roth) G. Don. J. Med. Food 2019, 22, 1041–1046. [Google Scholar] [CrossRef]
- Andreani, S.; Uehara, A.; Blagojević, P.; Radulović, N.; Muselli, A.; Baldovini, N. Key odorants of industrially-produced Helichrysum italicum subsp. italicum essential oil. Ind. Crop. Prod. 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Talić, S.; Odak, I.; Martinović Bevanda, A.; Crnjac, N.; Paštar, M. Helichrysum italicum (Roth) G. Don subsp. italicum from Herzegovina: Volatile composition, variations during seasons, total polyphenols, acetylcholinesterase inhibition and antioxidant activity. Croat. Chem. Acta 2019, 92, 69–77. [Google Scholar] [CrossRef]
- Genčić, M.S.; Aksić, J.M.; Živković Stošić, M.Z.; Đorđević, M.R.; Mladenović, M.Z.; Radulović, N.S. New neryl esters from Helichrysum italicum (Roth) G. Don (Asteraceae) essential oil. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical composition and antimicrobial activity of essential oil of Helichrysum italicum (Roth) G. Don fil. (Asteraceae) from Montenegro. Nat. Prod. Res. 2020, 34, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Mollova, S.; Fidan, H.; Antonova, D.; Bozhilov, D.; Stanev, S.; Kostova, I.; Stoyanova, A. Chemical composition and antimicrobial and antioxidant activity of Helichrysum italicum (Roth) G. Don subspecies essential oils. Turk. J. Agric. For. 2020, 44, 371–378. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Canini, A. Helichrysum italicum (Roth) G. Don essential oil: Composition and potential antineoplastic effect. S. Afr. J. Bot. 2020, 133, 222–226. [Google Scholar] [CrossRef]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don essential oil from Serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Arlorio, M.; Coisson, J.D.; Russo, M.T.; Pirisi, F.M.; Satta, M.; Cabras, P. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don ssp. michrophyllum (Willd) Nym. J. Agric. Food Chem. 2003, 51, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Mastelić, J.; Politeo, O.; Radosević, N. Composition and antimicrobial activity of Helichrysum italicum essential oil and its terpene and terpenoid fractions. Chem. Nat. Compd. 2005, 41, 35–39. [Google Scholar] [CrossRef]
- Rossi, P.G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; de Rocca Serra, D.; Bolla, M.J.M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Tundis, R.; Statti, G.A.; Conforti, F.; Bianchi, A.; Agrimonti, C.; Sacchetti, G.; Muzzoli, M.; Ballero, M.; Manichini, F.; Poli, F. Influence of enviromental factors on composition of volatile constituents and biological activity of Helichrysum italicum (Roth) Don (Asteraceae). Nat. Prod. Res. 2005, 19, 379–387. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. Antibacterial activity of Helichrysum italicum oil on vegetables and its mechanism of action. J. Food Process. Preserv. 2015, 39, 2663–267265. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.A.; Crisafi, G.; Musolino, A.D.; Procopio, F.; Alonzo, V. Modification of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett. Appl. Microbiol. 2004, 38, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Jukić, M.; Miloš, M. Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croat. Chem. Acta 2006, 79, 545–552. [Google Scholar]
- Sala, A.; Recio, M.; Giner, R.M.; Manez, S.; Tournier, H.; Schinella, G.; Rios, J.L. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Arbeiter, A.B.; Peeters, K.; Miklavčič Višnjevec, A.; Pražnikar, Z.J. HPLC-DAD-ESI-QTOF-MS determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Kramberger, K.; Pražnikar, Z.J.; Arbeiter, A.B.; Petelin, A.; Bandelj, D.; Kenig, S. A comparative study of the antioxidative effects of Helichrysum italicum and Helichrysum arenarium infusions. Antioxidants 2021, 10, 380. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Drapeau, J.; Fröhler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Lazdauskaite, Z. Helichrysum Mill. In Flora of Lithuania; Natkevicaite-Ivanauskiene, M., Jankeviciene, R., Lekavicius, A., Eds.; Publishing House Mokslas: Vilnius, Lithuania, 1980; Volume 6, pp. 54–56. (In Lithuanian) [Google Scholar]
- Pljevljakušić, D.; Bigović, D.; Janković, T.; Jelačić, S.; Šavikin, K. Sandy everlasting (Helichrysum arenarium (L.) Moench): Botanical, chemical and biological properties. Front. Plant Sci. 2018, 9, 1123. [Google Scholar] [CrossRef] [Green Version]
- Czinner, E.; Kéry, Á.; Hagymási, K.; Blázovics, A.; Lugasi, A.; Szőke, É.; Lemberkovics, É. Biologically active compounds of Helichrysum arenarium (L.) Moench. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 309–313. [Google Scholar] [CrossRef]
- Lemberkovics, É.; Czinner, E.; Szentmihalyi, K.; Balázs, A.; Szőke, É. Comparative evaluation of Helichrysi flos herbal extracts as dietary sources of plant polyphenols, and macro- and microelements. Food Chem. 2002, 78, 119–127. [Google Scholar] [CrossRef]
- Czinner, E.; Hagymási, K.; Blazovics, A.; Kéry, Á.; Szőke, É.; Lemberkovics, É. The in vitro effect of Helichrysi flos on microsomal lipid peroxidation. J. Ethnopharm. 2001, 77, 31–35. [Google Scholar] [CrossRef]
- Czinner, E.; Hagymási, K.; Blazovics, A.; Kéry, Á.; Szőke, É.; Lemberkovics, É. The in vitro antioxidant properties of Helichrysum arenarium (L.) Moench. J. Ethnopharm. 2000, 73, 437–443. [Google Scholar] [CrossRef]
- Kéry, Á.; Blazovics, A.; Fejes, S.; Nagy, E.; Lugasi, A.; Kursinszki, L.; Czinner, E.; Kristo, T.S.; Apati, P.; Balázs, A.; et al. Antioxidant activity of medical plants used in phytotherapy. Intern. J. Hort. Sci. 2001, 7, 28–35. [Google Scholar]
- Sroka, Z.; Kuta, I.; Cisowski, W.; Drys, A. Antiradical activity of hydrolyzed and non-hydrolyzed extracts from Helichrysi inflorescentia and its phenolic contents. Zeit. Naturforsch. 2004, 59c, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Rančić, A.; Soković, M.; Vukojević, J.; Simić, A.; Marin, P.; Duletić-Laušević, S. Chemical composition and antimicrobial activities of essential oils of Myrrhis odorata (L.) Scop, Hypericum perforatum L. and Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2005, 17, 341–345. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sağdiç, O.; Budak, Ü. Phenolic compounds and antioxidant and antimicrobial properties of Helichrysum species collected from Eastern Anatolia, Turkey. Turk. J. Biol. 2010, 34, 463–473. [Google Scholar]
- Grădinaru, A.C.; Silion, M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Helichrysum arenarium subsp. arenarium: Phenolic composition and antibacterial activity against lower respiratory tract pathogens. Nat. Prod. Res. 2014, 28, 2076–2080. [Google Scholar] [CrossRef]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. Moench through the pathway of anti-inflammation. Bioorg. Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef]
- Grinev, V.S.; Shirokov, A.A.; Navolokin, N.A.; Polukonova, N.V.; Kurchatova, M.N.; Durnova, N.A.; Bucharskaya, A.B.; Maslyakova, G.N. Polyphenolic compounds of a new biologically active extract from immortelle sandy flowers (Helichrysum arenarium (L.) Moench.). Russ. J. Bioorg. Chem. 2016, 42, 770–776. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sağdiç, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Moghadam, H.D.; Sani, A.; Sangatash, M.M. Inhibitory effect of Helichrysum arenarium essential oil on the growth of food contaminated microorganisms. J. Essent. Oil-Bear. Plants 2014, 17, 911–921. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.-M.; Vodnar, D.C.; Crişan, G.; Păltinean, R. Phytochemical analysis, antioxidant and antimicrobial activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori, M.; Poodineh, M.; Hakimzadeh, V. Composition of Helichrysum arenarium essential oil and antimicrobial activity against some food-born pathogens. Biotechnol. Ind. J. (BTAIJ) 2015, 11, 121–125. [Google Scholar]
- Eroğlu, H.; Hamzaoğlu, E.; Aksoy, A.; Budak, Ü.; Albayrak, S. Cytogenetic effects of Helichrysum arenarium in human lymphocytes cultures. Turk. J. Biol. 2010, 34, 253–259. [Google Scholar]
- Stanojević, D.; Ćomić, L.J.; Stefanović, O.; Solujić-Sukdoloak, S. In vitro synergistic antibacterial activity of Helichrysum arenarium, Inula helenium, Cichorium intybus and some preservatives. Ital. J. Food Sci. 2010, 22, 210–216. [Google Scholar]
- Morikawa, T.; Ninomiya, K.; Akaki, J.; Kakihara, N.; Kuramoto, H.; Matsumoto, Y.; Hayakawa, T.; Muraoka, O.; Wang, L.-B.; Wu, L.-J.; et al. Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum Arenarium. J. Nat. Med. 2015, 69, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Wang, L.-B.; Nakamura, S.; Ninomiya, K.; Ninomiya, K.; Yokoyama, E.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medicinal Flowers. XXVII. New flavanone and chalcone glycosides, Arenariumosides I, II, III, and IV, and tumor necrosis factor-α inhibitors from everlasting flowers of Helichrysum arenarium . Chem. Pharm. Bull. 2009, 57, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Meriçli, A.H.; Damadyan, B.; Çubukçu, B. Flavonoids of Turkish Helichrysum arenarium (L.) Moench (Asteraceae). Sci. Pharm. 1986, 54, 363–365. [Google Scholar]
- Czinner, E.; Kursinszki, L.; Baumann, D.; Hamburger, M.; Kéry, Á.; Szöke, É.; Lemberkovics, É. Phytochemical study of phenolic compounds from Helichrysi flos by LC-DAD-MS. In Natural Products in the New Millennium: Prospects and Industrial Application; Rauter, A.P., Palma, F.B., Justino, J., Araújo, M.E., dos Santos, S.P., Eds.; Proceedings of the Phytochemical Society of Europe; Springer: Dordrecht, The Netherlands, 2002; pp. 99–109. [Google Scholar]
- Eshbakova, K.A.; Aisa, H.A. Components of Helichrysum arenarium. Chem. Nat. Comp. 2009, 45, 929–930. [Google Scholar] [CrossRef]
- Yong, F.; Aisa, H.A.; Mukhamatkhanova, R.F.; Shamyanov, I.D.; Levkovich, M.G. New flavanone and other constituents of Helichrysum arenarium indigenous to China. Chem. Nat. Compd. 2011, 46, 872–875. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Gu, D.; Yili, A.; Sabir, G.; Aisa, H.A. Separation and purification of three flavonoids from Helichrysum arenarium (L.) Moench by HSCCC. Chromatographia 2009, 69, 963–967. [Google Scholar] [CrossRef]
- Liu, X.; King, X.; Li, G. A process to acquire essential oil by distillation concatenated liquid- liquid extraction and flavonoids by solid-liquid extraction simultaneously from Helichrysum arenarium (L.) Moench inflorescences under ionic liquid-microwave mediated. Sep. Purif. Technol. 2019, 209, 164–174. [Google Scholar] [CrossRef]
- Morikawa, T.; Wang, L.-B.; Ninomiya, K.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medicinal flowers. XXX. Eight new glycosides, everlastosides F-M, from the flowers of Helichrysum arenarium. Chem. Pharm. Bull. 2009, 57, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-B.; Morikawa, T.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medical flowers. XXVIII. Structures of five new glycosides, everlastosides A, B, C, D, and E, from the flowers of Helichrysum Arenarium. Heterocycles 2009, 78, 1235–1242. [Google Scholar]
- Bryksa-Godzisz, M.; Weglarz, Z.; Przyby, J. Phenolic compounds in yellow everlasting Helichrysum arenarium L. Moench growing wild in the middle part of the Bug river valley. Herba Pol. 2006, 52, 26–31. [Google Scholar]
- Czinner, E.; Lemberkovics, É.; Bihátsi-Karsai, É.; Vitányi, G.; Lelik, L. Composition of the essential oil from the inflorescence of Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2000, 12, 728–730. [Google Scholar] [CrossRef]
- Lemberkovics, É.; Czinner, A.; Balázs, E.; Bihátsi-Karsai, É.; Vitányi, G.; Lelik, L.; Bernáth, J.; Szőke, É. New data on composition of esssential oil from inflorescence of everlasting (Helichrysum arenarium (L.) Moench.). Acta Pharm. Hung. 2001, 71, 187–191. [Google Scholar]
- Judzentiene, A.; Butkiene, R. Chemical composition of the essential oils of wild Helichrysum arenarium (L.) with differently colored inflorescences from Eastern Lithuania. J. Essent. Oil Res. 2006, 18, 80–83. [Google Scholar] [CrossRef]
- Radušienė, J.; Judžentienė, A. Volatile composition of Helichrysum arenarium field accessions with differently coloured inflorescences. Biologia 2008, 54, 16–120. [Google Scholar] [CrossRef]
- Judžentienė, A.; Charkova, T.; Misiūnas, A. Chemical composition of the essential oils from Helichrysum arenarium (L.) plants growing in Lithuanian forests. J. Essent. Oil Res. 2019, 31, 305–311. [Google Scholar] [CrossRef]
- Bandeira Reidel, R.V.; Cioni, P.L.; Ruffoni, B.; Cervelli, C.; Pistelli, L. Aroma profile and essential oil composition of Helichrysum species. Nat. Prod. Commun. 2017, 12, 1507–1512. [Google Scholar]
- Stankov, S.; Fidan, H.; Petkova, N.; Stoyanova, A.; Dincheva, I.; Dogan, H.; Senkal, B.C.; Uskutoglu, T.; Bas, H.; Yilmaz, G. Phytochemical composition of Helichrysum arenarium (L.) Moench essential oil (aerial parts) from Turkey. Ukr. Food J. 2020, 9, 503–512. [Google Scholar] [CrossRef]
- Adams, R.P. Essential Oil Components by Quadrupole Gas Chromatography/Mass Spectrometry, 3rd ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2001. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley-WCH: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kalcher, K.; Svancara, I.; Buzuk, M.; Vytras, K.; Walcarius, A. Electrochemical sensors and biosensors based on heterogeneous carbon materials. Monatsh. Chem. 2009, 140, 861–889. [Google Scholar] [CrossRef]
- Judzentiene, A.; Garjonyte, R.; Budiene, J. Variability, toxicity, and antioxidant activity of Eupatorium cannabinum (hemp agrimony) essential oils. Pharm. Biol. 2016, 54, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastelić, J.; Politeo, O.; Jerković, I. Contribution to the analysis of the essential oil of Helichrysum italicum (Roth) G. Don.—Determination of ester bonded acids and phenols. Molecules 2008, 13, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Judzentiene, A.; Garjonyte, R.; Budiene, J. Toxic, radical scavenging, and antifungal activity of Rhododendron tomentosum H. essential oils. Molecules 2020, 25, 1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chu, L.; Liu, Y.; Wang, A.; Ji, B.; Wu, W.; Zhou, F.; Wei, Y.; Cheng, Q.; Cai, S.; et al. Analysis of the antioxidant capacities of flavonoids under different spectrophotometric assays using cyclic voltammetry and density functional theory. J. Agric. Food Chem. 2011, 59, 10277–10285. [Google Scholar] [CrossRef]
- Šeruga, M.; Tomac, I. Electrochemical behaviour of some chlorogenic acids and their characterization in coffee by square-wave voltammetry. Int. J. Electrochem. Sci. 2014, 9, 6134–6154. [Google Scholar]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical methods for total antioxidant capacity and its main contributors determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Couto, R.O. Flavonoid electrochemistry: A review on the electroanalytical applications. Revista Brasileira de Farmacognosia 2013, 23, 542–558. [Google Scholar] [CrossRef] [Green Version]
- Giacomelli, C.; Ckless, K.; Galato, D.; Miranda, F.S.; Spinelli, A. Electrochemistry of caffeic acid aqueous solutions with pH 2.0 to 8.5. J. Braz. Chem. Soc. 2002, 13, 332–338. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The use of biological assays to evaluate botanicals. Drug Inform. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Kadifkova Panovska, T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Idaomar, M.; El Hamss, R.; Bakkali, F.; Mezzoug, N.; Zhiri, A.; Baudoux, D.; Muñoz-Serrano, A.; Liemans, V.; Alonso-Moraga, A. Genotoxicity and antigenotoxicity of some essential oils evaluated by wing spot test of Drosophila melanogaster. Mutat. Res. 2002, 513, 61–68. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]


| Compound a | b RInLit | c RInexp | d RIpexp | H. aren. Infl. | H. aren. Leaves | H. ital. Infl. |
|---|---|---|---|---|---|---|
| α-Pinene * | 932 | 938 | 1035 | 0.3 ± 0.25 | 4.2 ± 1.15 | 6.5 ± 1.50 |
| Limonene | 1029 | 1030 | 1196 | tr. | 3.0 ± 0.50 | 1.6 ± 0.60 |
| 1,8-Cineole * | 1031 | 1032 | 1218 | 3.9 ± 0.60 | ||
| n-Nonanal | 1101 | 1103 | 1398 | 0.7 ± 0.20 | 10.4 ± 1.50 | |
| n-Decanal | 1202 | 1202 | 1503 | 0.9 ± 0.20 | 2.1 ± 2.0 | |
| Italicene | 1406 | 1406 | 2.2 ± 0.75 | |||
| trans-β-Caryophyllene * | 1419 | 1418 | 1608 | 5.4 ± 0.55 | 6.5 ± 0.55 | 3.3 ± 0.30 |
| γ-Curcumene | 1483 | 1486 | 21.5 ± 2.50 | |||
| β-Selinene | 1490 | 1490 | 1732 | 13.6 ± 1.65 | ||
| α-Selinene | 1498 | 1499 | 8.1 ± 0.55 | |||
| Lauric acid | 1577 | 2520 | 6.1 ± 1.35 | 2.0 ± 1.55 | 3.0 ± 0.15 | |
| β-Eudesmol | 1649 | 1650 | 2237 | 8.3 ± 0.35 | ||
| n-Tetradecanol | 1673 | 1672 | 1917 | 2.8 ± 0.35 | ||
| Eudesm-7-(11)-en-4-ol | 1700 | 1703 | 4.4 ± 0.40 | |||
| Myristic acid | 1741 | 2713 | 14.9 ± 1.05 | 8.7 ± 1.35 | 0.5 ± 0 | |
| Phytone | 1838 | 2113 | 4.4 ± 0.55 | 1.4 ± 0.85 | ||
| Pentadecylic acid | 1855 | 2820 | 2.1 ± 1.20 | 1.9 ± 0.95 | ||
| Palmitic acid | 1945 | 2911 | 23.8 ± 1.13 | 18.8 ± 0.70 | 0.2 ± 0.06 | |
| Methyl linolenate | 2075 | 2590 | 5.3 ± 0.75 | 2.7 ± 1.65 | ||
| n-Docosane | 2200 | 2200 | 3.8 ± 0.35 | 0.3 ± 0.10 | 0.3 ± 0.06 | |
| Average Total | 96.4 ± 1.52 | 99.1 ± 0.44 | 89.4 ± 0.15 |
| Identity | tR, min | Compound Formula | Molar Mass | m/z ESI+ (Da) | m/z ESI− (Da) |
|---|---|---|---|---|---|
| Bitalin A a | 3.1 | C13H14O3 | 218.25 | 219.028 | 214.943 |
| Bitalin A12-glucoside b | 3.2 | C19H24O8 | 380.4 | 381.084 | |
| Luteolin b | 6.7 | C15H10O6 | 286.24 | 289.093 | |
| 5,7-Dihydroxyphthalide b | 7.3 | C8H6O4 | 166.13 | 167.033 | |
| Kaempferol b | 7.4 | C15H10O6 | 286.24 | 289.090 | |
| Dihydrosyringin b | 8.2 | C17H26O9 | 374.39 | 375.093 | 371.008 |
| Triptophan b | 8.2 | C11H12N2O2 | 204.23 | 205.090 | |
| Caffeoylquinic (chlorogenic) acid b | 8.4 | C16H18O9 | 354.31 | 355.104 | 352.963 |
| Unknown b | 8.5 | 707.180 | 706.984 | ||
| Everlastoside E b | 8.6 | C19H28O11 | 432.4 | 433.135 | |
| Caffeic acid b | 8.7 | C9H8O4 | 180.16 | 181.049 | 180.933 |
| Apigenin-7-glucoside b | 8.7 | C21H20O10 | 432.38 | 433.130 | |
| Dimeric dihydrochalcone glycoside isomer b | 9.6 | C42H44O20 | 868 | 867.013 | |
| Naringenin b | 10.1 | C15H12O5 | 272.26 | 273.076 | |
| Naringenin glucoside isomer 1 b | 10.2 | C21H22O10 | 434.39 | 435.129 | 432.972 |
| Dimeric dihydrochalcone glycoside isomer b | 10.2 | C42H44O20 | 868 | 867.100 | |
| Syringin b | 10.5 | C17H24O9 | 272.37 | 273.076 | 273.076 |
| Dicaffeoylquinic acid a | 10.6 | C25H24O12 | 516.4 | 517.135 | 514.959 |
| Luteolin glycoside b | 10.8 | C21H20O11 | 448.37 | 449.109 | 446.950 |
| Naringenin glucoside isomer 2 b | 11.5 | C21H22O10 | 434.4 | 435.129 | 432.976 |
| Apigenin-7-O-gentiobioside/Apigenin-7,4′-di-O-β-glucoside b | 12.3 | C27H30O15 | 594.5 | 595.145 | 592.956 |
| Apigenin b | 13.7 | C15H10O5 | 270.24 | 271.060 | 268.944 |
| Arenol a,b | 18.0 | C21H24O7 | 388.41 | 389.160 | 387.015 |
| Arzanol a,b | 18.9 | C22H26O7 | 402.4 | 403.175 | 401.026 |
| Oleonolic acid a | 21.4 | C30H48O3 | 456.7 | 455.064 | |
| Heliarzanol b | 21.9 | C24H30O8 | 446.5 | 443.064 | |
| Resveratrol a | 22.7 | C14H12O3 | 228.25 | 226.88 | |
| Isosalipurposide b | 23.2 | C21H22O10 | 434.4 | 435.255 | 433.099 |
| β-Sitosterol b | 25.1 | C29H50O | 414.71 | 413.132 | |
| Quercetin 3-O-malonyl glucoside a | 31.3 | C24H22O15 | 550.4 | 550.63 |
| Equivalent, mmol/L | H. aren. Infl. EO (2021) | H. aren. Infl. EO (2020) | H. aren.Leaf EO (2020) | H. aren. Infl. Extract (2021) | H. aren.Leaf Extract (2021) | H. ital. Infl. EO |
|---|---|---|---|---|---|---|
| TROLOX | 0.27 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.001 | 6.13 ± 0.04 | 19.13 ± 0.04 | 0.35 ± 0.03 |
| Equivalent, mmol/L | H. aren. Infl. EO (2021) | H. aren. Infl. EO (2020) | H. aren. Leaf EO (2020) | H. aren. Infl. Extract (2021) | H. aren. Leaf Extact (2021) |
|---|---|---|---|---|---|
| TROLOX | 0.46 ± 0.01 | 0.40 ± 0.001 | 0.42 ± 0.01 | 1.96 ± 0.01 | 11.18 ± 0.002 |
| Artemia salina Nauplii Lethality | H. arenarium Inflorescence | H. arenarium Leaf | H. italicum Inflorescence |
|---|---|---|---|
| LC50, µg/mL | 23.42 | 21.97 | 15.99 |
| LC95, µg/mL | 83.82 | 82.66 | 43.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judzentiene, A.; Budiene, J.; Nedveckyte, I.; Garjonyte, R. Antioxidant and Toxic Activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don Essential Oils and Extracts. Molecules 2022, 27, 1311. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041311
Judzentiene A, Budiene J, Nedveckyte I, Garjonyte R. Antioxidant and Toxic Activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don Essential Oils and Extracts. Molecules. 2022; 27(4):1311. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041311
Chicago/Turabian StyleJudzentiene, Asta, Jurga Budiene, Irena Nedveckyte, and Rasa Garjonyte. 2022. "Antioxidant and Toxic Activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don Essential Oils and Extracts" Molecules 27, no. 4: 1311. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041311