Structure–Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Compounds 3–5
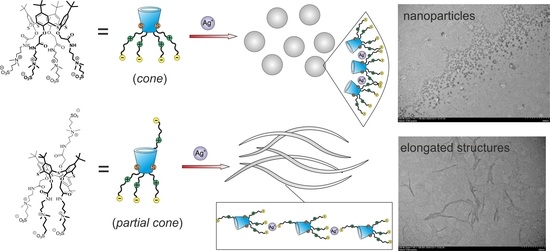
2.2. Self-Assembly of Water-Soluble Sulfobetaines 3–5
2.3. Cytotoxicity of Test Compounds 3–5 on Cancer and Non-Cancer Human Cell Lines
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of Compounds 3–5
3.3. Transmission Electron Microscopy (TEM)
3.4. Dynamic Light Scattering (DLS)
3.5. UV–Visible Spectroscopy
3.6. Сytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, J.M.; Wang, Y.Y.; Zhao, M.X.; Tan, C.P.; Li, Y.Q.; Le, X.Y.; Ji, L.N.; Mao, Z.W. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2018, 8, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T. Redox Regulation of Multidrug Resistance in Cancer Chemotherapy: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2009, 11, 99–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Avner, B.S.; Fialho, A.M.; Chakrabarty, A.M. Overcoming drug resistance in multi-drug resistant cancers and microorganisms. Bioengineered 2012, 3, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Flier, J.S.; Underhill, L.H.; Pastan, I.; Gottesman, M. Multiple-Drug Resistance in Human Cancer. N. Engl. J. Med. 1987, 316, 1388–1393. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Pillai, S.P.; Gentry, E.J.; Shankel, D.M. Multiple drug resistance. Med. Res. Rev. 1999, 19, 477–496. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [Green Version]
- Minagawa, Y.; Kigawa, J.; Itamochi, H.; Kanamori, Y.; Shimada, M.; Takahashi, M.; Terakawa, N. Cisplatin-resistant HeLa Cells Are Resistant to Apoptosis via p53-dependent and -independent Pathways. Jpn. J. Cancer Res. 1999, 90, 1373–1379. [Google Scholar] [CrossRef]
- Sameiyan, E.; Hayes, A.W.; Karimi, G. The effect of medicinal plants on multiple drug resistance through autophagy: A review of in vitro studies. Eur. J. Pharmacol. 2019, 852, 244–253. [Google Scholar] [CrossRef]
- Medvetz, D.A.; Hindi, K.M.; Panzner, M.J.; Ditto, A.J.; Yun, Y.H.; Youngs, W.J. Anticancer Activity of Ag(I) N-Heterocyclic Carbene Complexes Derived from 4,5-Dichloro-1H-Imidazole. Metal-Based Drugs 2008, 2008, 384010. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Consolidated Guidelines on Tuberculosis Module 4: Treatment Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, G.; Jang, Y.K.; Suh, J.S.; Kim, H.S.; Ahn, S.H.; Kim, T.J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix[n]arenes and Pillar[n]arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [Green Version]
- Yakimova, L.S.; Gilmanova, L.H.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Self-assembled fractal hybrid dendrites from water-soluble anionic (thia)calix[4 ]arenes and Ag+. J. Nanoparticle Res. 2017, 19, 173–183. [Google Scholar] [CrossRef]
- Grigoriev, M.; Babich, L. Use of Silver Nanoparticles in Treatment of Socially Significant Diseases. Key Eng. Mater. 2016, 683, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Lubick, N. Nanosilver toxicity: Ions, nanoparticles-or both? J. Environ. Sci. Technol. 2008, 42, 8617. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Qu, F.; Xu, H.; Lai, W.; Wang, A.Y.; Aguilar, Z.P.; Wei, H. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. BioMetals 2011, 25, 45–53. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- Cheng, H.B.; Zhang, Y.M.; Liu, Y.; Yoon, J. Turn-On Supramolecular Host-Guest Nanosystems as Theranostics for Cancer. Chem 2019, 5, 553–574. [Google Scholar] [CrossRef] [Green Version]
- Vavilova, A.A.; Nosov, R.V.; Yakimova, L.S.; Antipin, I.S.; Stoikov, I.I. Synthesis of Photo-Switchable Derivatives of p-tert-Butyl Thiacalix[4]arenes Containing Ethoxycarbonyl and 4-Amidoazobenzene Fragments in the Lower Rim Substituents. Macroheterocycles 2013, 6, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Gorbatchuk, V.V.; Gatiatulin, A.K.; Ziganshin, M.A.; Gubaidullin, A.T.; Yakimova, L.S. Unusually high efficiency of β-cyclodextrin clathrate preparation by water-free solid-phase guest exchange. J. Phys. Chem. B 2013, 117, 14544–14556. [Google Scholar] [CrossRef] [PubMed]
- Puplampu, J.B.; Yakimova, L.S.; Vavilova, A.A.; Fayzullin, D.A.; Zuev, Y.F.; Stoikov, I.I. Synthesis of p-tert-butylthiacalix[4]arenes functionalized with tris(2-aminoethyl)amine fragments at the lower rim and their interaction with model lipid membranes. Macroheterocycles 2014, 7, 337–344. [Google Scholar] [CrossRef]
- Gorbatchuk, V.V.; Savelyeva, L.S.; Ziganshin, M.A.; Antipin, I.S.; Sidorov, V.A. Molecular recognition of organic guest vapor by solid adamantylcalix[4]arene. Russ. Chem. Bull. 2004, 53, 60–65. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, Y.O.; Bhalla, V.; Kumar, M.; Kim, J.S. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 2014, 43, 4824. [Google Scholar] [CrossRef]
- Yakimova, L.; Vavilova, A.; Shibaeva, K.; Sultanaev, V.; Mukhametzyanov, T.; Stoikov, I. Supramolecular approaches to the formation of nanostructures based on phosphonate-thiacalix[4]arenes, their selective lysozyme recognition. Colloids Surf. A Physicochem. Eng. 2021, 611, 125897. [Google Scholar] [CrossRef]
- Ziganshin, M.A.; Yakimova, L.S.; Khayarov, K.R.; Gorbatchuk, V.V.; Vysotsky, M.O.; Böhmer, V. Guest exchange in dimeric capsules of a tetraurea calix[4]arene in the solid state. Chem. Commun. 2006, 37, 3897–3899. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Padnya, P.L.; Kunafina, A.F.; Nugmanova, A.R.; Stoikov, I.I. Sulfobetaine derivatives of thiacalix[4]arene: Synthesis and supramolecular self-assembly of submicron aggregates with AgI cations. Mendeleev Commun. 2019, 29, 86–88. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.L.; Andreyko, E.A.; Mostovaya, O.A.; Rizvanov, I.K.; Stoikov, I.I. The Synthesis of New Amphiphilic P-Tert-Butylthiacalix[4]Arenes Containing Peptide Fragments and Their Interaction with DNA. Org. Biomol. Chem. 2015, 13, 5894–5904. [Google Scholar] [CrossRef] [PubMed]
- Sýkora, J.; Himl, M.; Stibor, I.; Císařová, I.; Lhoták, P. Unique self-assembly patterns based on thiacalix[4]arene–silver interactions. Tetrahedron 2007, 63, 2244–2248. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, M.; Hundal, G. Synthesis, NMR, X-ray structural analyses and complexation studies of new Ag+ selective calix[4]arene based dipodal hosts—a co-complexation of neutral and charged species. Tetrahedron 2004, 60, 5393–5405. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B 2014, 117, 354–359. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Kim, E.S.; Choi, Y.J.; Kwon, D.N.; Kim, J.H. Reduced graphene oxide–silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257–6276. [Google Scholar] [CrossRef] [Green Version]
- Ayoup, M.S.; Wahby, Y.; Abdel-Hamid, H.; Ramadan, E.S.; Teleb, M.; Abu-Serie, M.M.; Noby, A. Design, synthesis and biological evaluation of novel α-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. Eur. J. Med. Chem. 2019, 168, 340–356. [Google Scholar] [CrossRef]
- Andreyko, E.A.; Padnya, P.L.; Daminova, R.R.; Stoikov, I.I. Supramolecular “containers”: Self-assembly and functionalization of thiacalix[4]arenes for recognition of amino- and dicarboxylic acids. RSC Adv. 2014, 4, 3556–3565. [Google Scholar] [CrossRef]
- Iki, N.; Narumi, F.; Fujimoto, T.; Morohashi, N.; Miyano, S. Selective Synthesis of Three Conformational Isomers of Tetrakis[(Ethoxycarbonyl)Methoxy]Thiacalix[4]Arene and Their Complexation Properties towards Alkali Metal Ions. J. Chem. Soc. Perkin Trans. 2 1998, 2, 2745–2750. [Google Scholar] [CrossRef]





| Test Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| M-HeLa | HuTu 80 | MCF-7 | Chang Liver | |
| 3 | >5000 | >5000 | >5000 | >5000 |
| 3/Ag+ | >5000 | >5000 | >5000 | >5000 |
| 4 | >500 | >500 | >500 | >500 |
| 4/Ag+ | 38.9 ± 2.6 | >500 | >500 | 85.1 ± 6.4 |
| 5 | >500 | >500 | >500 | >500 |
| Imatinib mesylate | 84.7 ± 6.3 | 288 ± 23 | 207 ± 17 | 102 ± 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimova, L.; Kunafina, A.; Nugmanova, A.; Padnya, P.; Voloshina, A.; Petrov, K.; Stoikov, I. Structure–Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines. Molecules 2022, 27, 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041364
Yakimova L, Kunafina A, Nugmanova A, Padnya P, Voloshina A, Petrov K, Stoikov I. Structure–Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines. Molecules. 2022; 27(4):1364. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041364
Chicago/Turabian StyleYakimova, Luidmila, Aisylu Kunafina, Aigul Nugmanova, Pavel Padnya, Alexandra Voloshina, Konstantin Petrov, and Ivan Stoikov. 2022. "Structure–Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines" Molecules 27, no. 4: 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041364