Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations
Abstract
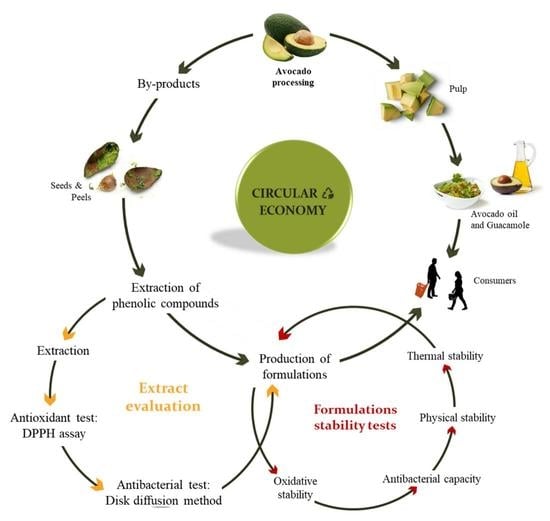
:1. Introduction
2. Results and Discussion
2.1. Extraction and Characterisation of the Avocado Peel Extract
2.2. W/O and O/W Formulations Stability
3. Methods and Materials
3.1. Chemicals and Reagents
3.2. Methods
3.2.1. Extraction of Phenolic Compounds from Avocado Peels
3.2.2. Characterisation of Avocado Peel Extract
3.2.3. Moisturising Cream Production
3.2.4. Stability Tests
Accelerated thermal stability test: Temperature variation
Accelerated physical stability test: Centrifugation
Determination of peroxide value (PV)
Antibacterial assay of the formulations: Agar well diffusion method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APE | Avocado Peel Extract |
| O/W | Oil-in-water |
| PHEN | Phenoxyethanol |
| PV | Peroxide Value |
| UV | Ultraviolet |
| W/O | Water-in-oil |
References
- Thibane, V.S.; Ndhlala, A.R.; Abdelgadir, H.A.; Finnie, J.F.; Van Staden, J. The Cosmetic Potential of Plants from the Eastern Cape Province Traditionally Used for Skincare and Beauty. S. Afr. J. Bot. 2019, 122, 475–483. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C.; Sousa Lobo, J.M.; Sousa, E.; Almeida, I.F. Trends in the Use of Marine Ingredients in Anti-Aging Cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Kim, M.H.; Jeong, J.S.; Choi, S.H. Analysis of Characteristics and Risk Factors of Surgical Site Infection after Coronary Artery Bypass Graft. Korean J. Healthc. Infect. Control Prev. 2016, 21, 57. [Google Scholar] [CrossRef] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Brunt, E.G.; Burgess, J.G. The Promise of Marine Molecules as Cosmetic Active Ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Composition of Creams for Skin Care. In Cosmetic Creams; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 131–173. [CrossRef]
- Costa, R.; Santos, L. Delivery Systems for Cosmetics—From Manufacturing to the Skin of Natural Antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Microencapsulation of Caffeic Acid and Its Release Using a w/o/w Double Emulsion Method: Assessment of Formulation Parameters. Dry. Technol. 2019, 37, 950–961. [Google Scholar] [CrossRef]
- Lodén, M. The Clinical Benefit of Moisturizers. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 672–688. [Google Scholar] [CrossRef]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-Aging and Sunscreens: Paradigm Shift in Cosmetics. Adv. Pharm. Bull. 2019, 2019, 348–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, K. Nutraceuticals and Skin Health: Key Benefits and Protective Properties. J. Aesthetic Nurs. 2018, 7 (Suppl. 1), 35–40. [Google Scholar] [CrossRef]
- Deckner, G. Antioxidants: Powerful Skin Care Actives & Stabilizers. Available online: https://knowledge.ulprospector.com/2963/pcc-antioxidants-powerful-skin-care-actives-stabilizers/ (accessed on 9 December 2021).
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry By-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Faria-silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Margarida, H.; Simões, S. Trends in Food Science & Technology Feeding the Skin: A New Trend in Food and Cosmetics Convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Zein, A.; Tlais, A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–774. [Google Scholar] [CrossRef] [Green Version]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Lefebvre, G.; Jiménez, E.; Cabañas, B. Environment, Energy and Climate Change II—Energies from New Resources and the Climate Change; Springer: New York, NY, USA, 2016; pp. 199–227. [Google Scholar]
- FAO. Avocado World Production. Available online: https://www.fao.org/faostat/en/#compare (accessed on 18 January 2022).
- Lye, H.S.; Ong, M.K.; Teh, L.K.; Chang, C.C.; Wei, L.K. Avocado. In Valorization of Fruit Processing By-Products; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 67–93. [Google Scholar] [CrossRef]
- Cowan, A.; Wolstenholme, B.N. Avocado. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 294–300. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive Identification of Bioactive Compounds of Avocado Peel by Liquid Chromatography Coupled to Ultra-High-Definition Accurate-Mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the Antioxidant and Antimicrobial Response of the Combined Effect of Nisin and Avocado Byproducts. LWT-Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- Daiuto, É.R.; Tremocoldi, M.A.; Matias De Alencar, S.; Vieites, R.L.; Minarelli, P.H. Chemical Composition and Antioxidant Activity of the Pulp, Peel and by Products of Avocado ’Hass. Rev. Bras. Frutic. 2014, 36, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Manuela, M.; Pintado, E.; Aguilar, C.N. Avocado By-Products: Nutritional and Functional Properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Akan, S. Phytochemicals in Avocado Peel and Their Potential Uses. Food Health 2021, 7, 138–149. [Google Scholar] [CrossRef]
- Morcuende, D.; Kylli, P.; Est, M. Avocado (Persea Americana Mill.) Phenolics. In Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar]
- Raymond Chia, T.W.; Dykes, G.A. Antimicrobial Activity of Crude Epicarp and Seed Extracts from Mature Avocado Fruit (Persea Americana) of Three Cultivars. Pharm. Biol. 2010, 48, 753–756. [Google Scholar] [CrossRef]
- Rotta, E.M.; de Morais, D.R.; Biondo, P.B.F.; dos Santos, V.J.; Matsushita, M.; Visentainer, J.V. Uso Da Casca Do Abacate (Persea Americana) Na Formulação de Chá: Um Produto Funcional Contendo Compostos Fenólicos e Atividade Antioxidante. Acta Sci.-Technol. 2016, 38, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Colombo, R.; Papetti, A. Avocado (Persea Americana Mill.) by-Products and Their Impact: From Bioactive Compounds to Biomass Energy and Sorbent Material for Removing Contaminants. A Review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and Other Polar Compounds in the Edible Part and by-Products of Avocado. LWT-Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Páramos, P.R.S.; Granjo, J.F.O.; Corazza, M.L.; Matos, H.A. Extraction of High Value Products from Avocado Waste Biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Céspedes-Acuña, C.; Silva, F.L.; Alarcón-Enos, J. Improvement of the Polyphenol Extraction from Avocado Peel by Assisted Ultrasound and Microwaves. J. Food Process Eng. 2019, 42, e13197. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive Characterization of Persea Americana Mill. by-Products: A Rich Source of Inherent Antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.; García, A.; Aranda, M.; Saéz, V.; Zúñiga, F.; Alarcón, J.; Avello, M.; Pastene, E. One-Step Purification of Two Semi-Synthetic Epicatechin Adducts Prepared from Avocado Peels Procyanidins by Centrifugal Partition Chromatography and Evaluation of Their Anti-Inflammatory Effects on Adenocarcinoma Gastric Cells Infected with Helicobacter P. J. Chil. Chem. Soc. 2018, 63, 4222–4228. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Pérez, Y.M.; Cedeño-Linares, E.; Norman-Montenegro, O.; Ruz-Sanjuan, V.; Mondeja-Rivera, Y.; Hernández-Monzón, A.M.; González-Bedia, M.M. Prediction of the Physical Stability and Quality of O/W Cosmetic Emulsions Using Full Factorial Design [Predicción de La Estabilidad Física y Calidad de Emulsiones Cosméticas O/W Mediante Diseño Factorial Completo]. J. Pharm. Pharmacogn. Res. 2021, 9, 98–112. [Google Scholar]
- Colucci, G.; Santamaria-Echart, A.; Silva, S.C.; Fernandes, I.P.M.; Sipoli, C.C.; Barreiro, M.F.; Arpicco, S.; Bergonzi, M.C. Development of Water-in-Oil Emulsions as Delivery Vehicles and Testing with a Natural Antimicrobial Extract. Molecules 2020, 25, 2105. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of Cosmetic Active Ingredients for Topical Application—A Review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef]
- Thakore, K.N. Butylated Hydroxyanisole. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 581–582. [Google Scholar] [CrossRef]
- Vergara, C.; von Baer, D.; Hermosín, I.; Ruiz, A.; Hitschfeld, M.A.; Castillo, N.; Mardones, C. Anthocyanins That Confer Characteristic Color to Red Copihue Flowers (Lapageria Rosea). J. Chil. Chem. Soc. 2009, 54, 194–197. [Google Scholar] [CrossRef]
- Cox, K.A.; McGhie, T.K.; White, A.; Woolf, A.B. Skin Colour and Pigment Changes during Ripening of “Hass” Avocado Fruit. Postharvest Biol. Technol. 2004, 31, 287–294. [Google Scholar] [CrossRef]
- Turner, R.; McLean, C.H.; Silvers, K.M. Are the Health Benefits of Fish Oils Limited by Products of Oxidation? Nutr. Res. Rev. 2006, 19, 53–62. [Google Scholar] [CrossRef]
- Sampels, S. Oxidation and Antioxidants in Fish and Meat from Farm to Fork. In Food Industry; Muzzalupo, I., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef] [Green Version]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- El Gengaihi, S. Antioxidant Activity of Phenolic Compounds from Different Grape Wastes. J. Food Process Technol. 2014, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential Application of Grape (Vitis Vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Nalur, S.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]





| Extraction Time | |||
| 1.5 h | 3 h | 4 h | |
| Yield (%) | |||
| 19.49 ± 0.56 | 22.88 ± 1.06 | 22.24 ± 1.80 | |
| Antioxidant Assay | |||
| DPPH Inhibition (%) | 93.92 ± 1.29 | 92.89 ± 0.77 | 91.18 ± 0.48 |
| IC50 (µgsample∙mLDPPH−1) | 37.30 ± 1.00 | 38.23 ± 2.33 | 38.79 ± 0.70 |
| Antibacterial Assay–Inhibition halos (mm) | |||
| E. coli | <5.0 | <5.0 | <5.0 |
| S. aureus | 13.0 ± 0.0 | 12.7 ± 0.9 | 10.0 ± 0.8 |
| S. epidermidis | 14.0 ± 0.8 | 12.7 ± 0.5 | 9.3 ± 0.5 |
| Peroxide Values (mEq/gformulation) | ||||||
|---|---|---|---|---|---|---|
| O/W Emulsion | W/O Emulsion | |||||
| Formulation | F1 | F2 | F4 | F1 | F2 | F4 |
| 7th day | 0.294 ± 0.5 × 10−3 | 0.049 ± 4.0 × 10−3 | 0.222 ± 1.3 × 10−3 | 0.277 ± 0.7 × 10−3 | 0.177 ± 3.1 × 10−3 | 0.464 ± 3.0 × 10−3 |
| 14th day | 0.379 ± 2.0 × 10−3 | 0.057 ± 5.1 × 10−3 | 0.244 ± 4.3 × 10−3 | 0.382 ± 1.8 × 10−3 | 0.206 ± 3.2 × 10−3 | 0.536 ± 3.1 × 10−3 |
| 21th day | n.d. | n.d. | 0.063 ± 1.6 × 10−2 | n.d. | n.d. | 0.353 ± 4.1 × 10−3 |
| PV increase from the 7th to the 14th day (%) | 29 | 15 | 10 | 38 | 16 | 15 |
| Antibacterial Assay Inhibition Halos (mm) of the Formulations | ||||||||
|---|---|---|---|---|---|---|---|---|
| O/W Emulsion | W/O Emulsion | |||||||
| F1 | F3 | F4 | F5 | F1 | F3 | F4 | F5 | |
| E. coli | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| S. aureus | <5.0 | 16.7 ± 1.3 | 20.7 ± 0.5 | 20.7 ± 0.5 | 11.0 ± 0.0 | 12.7 ± 0.5 | 8.7 ± 0.5 | 10.3 ± 0.5 |
| S. epidermidis | <5.0 | 17.3 ± 0.5 | 18.3 ± 1.3 | 22.0 ± 1.4 | 16.3 ± 0.9 | 17.0 ± 0.0 | 13.7 ± 0.9 | 15.0 ± 0.0 |
| pH | ||||||||
| 5.5 | 5.5 | 5.5 | 5.5 | 6 | 6 | 5.5 | 5.5 | |
| Emulsion | Ingredient | Function | Percentage (%) | |||
|---|---|---|---|---|---|---|
| W/O | Aqueous phase | |||||
| Ultrapure water | Solvent | 60.0 | ||||
| Glycerin | Humectant | 10.0 | ||||
| Sodium lactate 50% solution | Humectant | 2.0 | ||||
| Glycerol Monostearate | Emulsifier | 2.0 | ||||
| Cocamidopropyl betaine | Emulsifier | 4.0 | ||||
| Oil Phase | ||||||
| Shea Butter | Emollient | 14.0 | ||||
| Beeswax | Emollient | 8.0 | ||||
| O/W | Aqueous phase | |||||
| Ultrapure water | Solvent | 70.0 | ||||
| Glycerin | Humectant | 7.6 | ||||
| Sodium lactate 50% solution | Humectant | 3.2 | ||||
| Xanthan Gum | Thickening Agent | 0.8 | ||||
| Oil Phase | ||||||
| Coconut oil | Emollient | 7.6 | ||||
| Beeswax | Emollient | 6.8 | ||||
| Soy lecithin | Emulsifier | 2.5 | ||||
| Cocamidopropyl betaine | Emulsifier | 1.5 | ||||
| Additives | Formulation | |||||
| F1 | F2 | F3 | F4 | F5 | ||
| BHT | - | 0.5 | - | - | - | |
| PHEN | - | - | 1.0 | - | 0.5 | |
| APE | - | - | - | 0.5 | 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27061782
Ferreira SM, Falé Z, Santos L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules. 2022; 27(6):1782. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27061782
Chicago/Turabian StyleFerreira, Sara M., Zizina Falé, and Lúcia Santos. 2022. "Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations" Molecules 27, no. 6: 1782. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27061782