Secondary Metabolism Rearrangements in Linum usitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall
Abstract
:1. Introduction
2. Results
2.1. Preparation of Chitosan Oligosaccharides Elicitors
2.1.1. Chitosan Degradation and Chitosan Oligosaccharides Preparation
2.1.2. Structural Characterization of Chitosan Oligomers by ESI-HRMS and NMR Analysis
2.2. Defense Responses of Flax Seedlings Activated by Chitosan Oligosaccharides (COS) Elicitation
2.2.1. Phenotypic Observations
2.2.2. UPLC-MS Data and Metabolite Identification
2.2.3. Metabolic Changes in Flax Seedling Root and Shoot Tissues in Response to COS Applied Locally at Roots
3. Discussion
4. Materials and Methods
4.1. Chitosan Oligosaccharides Preparation and Analysis
4.1.1. Preparation of COS Oligosaccharides
4.1.2. Electrospray-Ionization High Resolution Mass Spectrometry (ESI-HRMS) Analysis
4.1.3. NMR Analysis
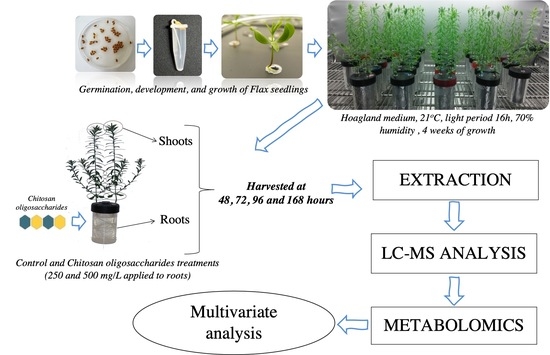
4.2. Plant Material, Growth and COS Oligosaccharides Treatments
4.3. Metabolite Extractions
4.4. Metabolite Analysis by LC/MS
4.4.1. Samples Preparation
4.4.2. UPLC-MS Data Acquisition
4.4.3. UPLC-MS Data Processing
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A


References
- Zuk, M.; Richter, D.; Matuła, J.; Szopa, J. Linseed, the multipurpose plant. Ind. Crops Prod. 2015, 75, 165–177. [Google Scholar] [CrossRef]
- Cullis, C.A. Flax. In Oilseeds; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 275–295. ISBN 978-3-540-34388-2. [Google Scholar]
- Hall, C.; Tulbek, M.C.; Xu, Y. Flaxseed. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 1–97. [Google Scholar]
- Baumann, B.B. The botanical aspects of ancient Egyptian embalming and burial. Econ. Bot. 1960, 14, 84–104. [Google Scholar] [CrossRef]
- Stitt, P.A. History of fl ax: 9000 years ago to 1986. In Proceedings of the 55th Flax Institute of the United States, Fargo, ND, USA, 26–28 January 1994. [Google Scholar]
- Palla, A.H.; Khan, N.A.; Bashir, S.; ur-Rehman, N.; Iqbal, J.; Gilani, A.-H. Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J. Ethnopharmacol. 2015, 160, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, C.; Duan, Y.; Deng, Q.; Peng, D.; Zhang, H.; Ma, H. The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: A review. Trends Food Sci. Technol. 2021, 118, 252–260. [Google Scholar] [CrossRef]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current Uses and Future Applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Maclay, W.D.; Matchett, J.R.; Pollack, M. Industrial Utilization of Seed Oils. Econ. Bot. 1963, 17, 23–30. [Google Scholar] [CrossRef]
- Mallégol, J.; Lemaire, J.; Gardette, J.-L. Drier influence on the curing of linseed oil. Prog. Org. Coat. 2000, 39, 107–113. [Google Scholar] [CrossRef]
- Yasmeen, M.; Nisar, S.; Tavallali, V.; Khalid, T. A review of phytochemicals and uses of flaxseed. Int. J. Chem. Biochem. Sci. 2018, 13, 70–75. [Google Scholar]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Mannucci, A.; Castagna, A.; Santin, M.; Serra, A.; Mele, M.; Ranieri, A. Quality of flaxseed oil cake under different storage conditions. LWT 2019, 104, 84–90. [Google Scholar] [CrossRef]
- Piotrowski, S.; Carus, M. Ecological benefits of hemp and flax cultivation and products. Nova Inst. 2011, 5, 1–6. [Google Scholar]
- Muir, A.D.; Westcott, N.D. Flax: The Genus Linum; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-43750-6. [Google Scholar]
- Boba, A.; Kostyn, K.; Kostyn, A.; Wojtasik, W.; Dziadas, M.; Preisner, M.; Szopa, J.; Kulma, A. Methyl salicylate level increase in flax after fusarium oxysporum infection Is associated with phenylpropanoid pathway activation. Front. Plant Sci. 2016, 7, 1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant–pathogen interactions. Front. Plant Sci. 2014, 5, 228:1–228:8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Kostyn, K.; Czemplik, M.; Kulma, A.; Bortniczuk, M.; Skała, J.; Szopa, J. Genes of phenylpropanoid pathway are activated in early response to Fusarium attack in flax plants. Plant Sci. 2012, 190, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Tchoumtchoua, J.; Mathiron, D.; Pontarin, N.; Gagneul, D.; van Bohemen, A.-I.; Otogo N’nang, E.; Mesnard, F.; Petit, E.; Fontaine, J.-X.; Molinié, R.; et al. Phenolic profiling of flax highlights contrasting patterns in winter and spring varieties. Molecules 2019, 24, 4303. [Google Scholar] [CrossRef] [Green Version]
- Stadnik, M.J.; de Freitas, M.B. Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115221. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Xing, R.; Liu, S.; Li, P. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meénard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.-C.; Kauffmann, S. Β-1,3 glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wang, W.; Liu, Q.; Li, J.; Yin, H. The application of chito/chitin oligosaccharides as plant vaccines. In Oligosaccharides of Chitin and Chitosan: Bio-Manufacture and Applications; Zhao, L., Ed.; Springer: Singapore, 2019; pp. 289–323. ISBN 9789811394027. [Google Scholar]
- Chatelain, P.G.; Pintado, M.E.; Vasconcelos, M.W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014, 215–216, 134–140. [Google Scholar] [CrossRef]
- Nars, A.; Rey, T.; Lafitte, C.; Vergnes, S.; Amatya, S.; Jacquet, C.; Dumas, B.; Thibaudeau, C.; Heux, L.; Bottin, A.; et al. An experimental system to study responses of Medicago truncatula roots to chitin oligomers of high degree of polymerization and other microbial elicitors. Plant Cell Rep. 2013, 32, 489–502. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Sarma, P.V.S.R.N.; Ankati, S.; Bhuvanachandra, B.; Podile, A.R. Elicitation of defense response by transglycosylated chitooligosaccharides in rice seedlings. Carbohydr. Res. 2021, 510, 108459. [Google Scholar] [CrossRef]
- Khan, W.; Costa, C.; Souleimanov, A.; Prithiviraj, B.; Smith, D.L. Response of Arabidopsis thaliana roots to lipo-chitooligosaccharide from Bradyrhizobium japonicum and other chitin-like compounds. Plant Growth Regul. 2011, 63, 243–249. [Google Scholar] [CrossRef]
- Gamir, J.; Minchev, Z.; Berrio, E.; García, J.M.; De Lorenzo, G.; Pozo, M.J. Roots drive oligogalacturonide-induced systemic immunity in tomato. Plant Cell Environ. 2021, 44, 275–289. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Paulin, M.; Miot-Sertier, C.; Dutilh, L.; Brasselet, C.; Delattre, C.; Pierre, G.; Dubessay, P.; Michaud, P.; Doco, T.; Ballestra, P.; et al. Brettanomyces bruxellensis Displays Variable Susceptibility to Chitosan Tteatment in Wine. Front. Microbiol. 2020, 11, 571067. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Dual extraction of crustacean and fungal chitosan from a single Mucor circinelloides Fermentation. Fermentation 2020, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Oberemko, A.; Salaberria, A.M.; Saule, R.; Saulis, G.; Kaya, M.; Labidi, J.; Baublys, V. Physicochemical and in vitro cytotoxic properties of chitosan from mushroom species (Boletus bovinus and Laccaria laccata). Carbohydr. Polym. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Jia, Z.; Shen, D. Effect of reaction temperature and reaction time on the preparation of low-molecular-weight chitosan using phosphoric acid. Carbohydr. Polym. 2002, 49, 393–396. [Google Scholar] [CrossRef]
- No, H.K.; Nah, J.W.; Meyers, S.P. Effect of time/temperature treatment parameters on depolymerization of chitosan. J. Appl. Polym. Sci. 2003, 87, 1890–1894. [Google Scholar] [CrossRef]
- Knill, C.; Kennedy, J.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.; Williams, H. Acid hydrolysis of commercial chitosans. J. Chem. Technol. Biotechnol. 2005, 80, 1291–1296. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan I: Kinetics of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef]
- Einbu, A.; Vårum, K.M. Depolymerization and De-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 2007, 8, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of chitosan by acids. Sci. World J. 2013, 2013, e508540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhang, J.; Bu, F.; Xia, W. Determination of chitosan with a modified acid hydrolysis and HPLC method. Carbohydr. Res. 2013, 366, 50–54. [Google Scholar] [CrossRef]
- Horowitz, S.T.; Roseman, S.; Blumenthal, H.J. The Preparation of Glucosamine Oligosaccharides. I. Separation. J. Am. Chem. Soc. 1957, 79, 5046–5049. [Google Scholar] [CrossRef]
- Voxeur, A.; Habrylo, O.; Guénin, S.; Miart, F.; Soulié, M.-C.; Rihouey, C.; Pau-Roblot, C.; Domon, J.-M.; Gutierrez, L.; Pelloux, J.; et al. Oligogalacturonide production upon Arabidopsis thaliana–Botrytis cinerea interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 19743–19752. [Google Scholar] [CrossRef] [Green Version]
- Domard, A.; Cartier, N. Glucosamine oligomers: 1. preparation and characterization. Int. J. Biol. Macromol. 1989, 11, 297–302. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef]
- Blum, A.; Bressan, M.; Zahid, A.; Trinsoutrot-Gattin, I.; Driouich, A.; Laval, K. Verticillium wilt on fiber flax: Symptoms and pathogen development In planta. Plant Dis. 2018, 102, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Hamel, L.-P.; Beaudoin, N. Chitooligosaccharide sensing and downstream signaling: Contrasted outcomes in pathogenic and beneficial plant–microbe interactions. Planta 2010, 232, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Guarnizo, N.; Oliveros, D.; Murillo-Arango, W.; Bermúdez-Cardona, M.B. Oligosaccharides: Defense inducers, their recognition in plants, commercial uses and perspectives. Molecules 2020, 25, 5972. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, W.; Preisner, M.; Boba, A.; Kostyn, K.; Dymińska, L.; Hanuza, J.; Szopa, J.; Kulma, A. Rearrangement of cell wall polymers in flax infected with a pathogenic strain of Fusarium culmorum. Physiol. Mol. Plant Pathol. 2020, 110, 101461. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Hemmati, S.; Klaes, M.; Konuklugil, B.; Mohagheghzadeh, A.; Ionkova, I.; Fuss, E.; Wilhelm Alfermann, A. Lignans in flowering aerial parts of Linum species—Chemodiversity in the light of systematics and phylogeny. Phytochemistry 2010, 71, 1714–1728. [Google Scholar] [CrossRef]
- Ryan, C.A.; Farmer, E.E. Oligosaccharide signals in plants: A current assessment. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 651–674. [Google Scholar] [CrossRef]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Ahmad, W.; Zahir, A.; Nadeem, M.; Garros, L.; Drouet, S.; Renouard, S.; Doussot, J.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Enhanced production of lignans and neolignans in chitosan-treated flax (Linum usitatissimum L.) cell cultures. Process Biochem. 2019, 79, 155–165. [Google Scholar] [CrossRef]
- Esmaeilzadeh Bahabadi, S.; Sharifi, M.; Safaie, N.; Behmanesh, M. Enhancement of lignan and phenylpropanoid compounds production by chitosan in Linum album cell culture. Iran. J. Plant Biol. 2012, 4, 13–26. [Google Scholar]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Marschner, P.; Rengel, Z. Chapter 13—Effect of Internal and external factors on root rrowth and development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 331–346. ISBN 978-0-12-384905-2. [Google Scholar]
- Jia, X.; Rajib, M.R.; Yin, H. Recognition pattern, functional mechanism and application of chitin and chitosan oligosaccharides in sustainable agriculture. Curr. Pharm. Des. 2020, 26, 3508–3521. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and chitosan fragments responsible for plant elicitor and growth stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Zhang, X.Q.; Yu, Y.; Xing, R.E.; Liu, S.; Li, P.C. Effect of chitin and chitosan hexamers on growth and photosynthetic characteristics of wheat seedlings. Photosynthetica 2020, 58, 819–826. [Google Scholar] [CrossRef]
- Cabrera, J.-C.; Boland, A.; Cambier, P.; Frettinger, P.; Van Cutsem, P. Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 2010, 20, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Basa, S.; Nampally, M.; Honorato, T.; Das, S.N.; Podile, A.R.; El Gueddari, N.E.; Moerschbacher, B.M. The pattern of acetylation defines the priming activity of chitosan tetramers. J. Am. Chem. Soc. 2020, 142, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohydr. Polym. 2016, 138, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Liu, S.; Zou, P.; Xing, R.; Yu, H.; Chen, X.; Qin, Y.; Li, P. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of Wheat seedlings under salt stress. J. Agric. Food Chem. 2017, 65, 501–509. [Google Scholar] [CrossRef]
- Xie, X.; Yan, Y.; Liu, T.; Chen, J.; Huang, M.; Wang, L.; Chen, M.; Li, X. Data-independent acquisition proteomic analysis of biochemical factors in rice seedlings following treatment with chitosan oligosaccharides. Pestic. Biochem. Physiol. 2020, 170, 104681. [Google Scholar] [CrossRef]
- Yin, H.; Kjaer, A.; Fretté, X.C.; Du, Y.; Christensen, L.P.; Jensen, M.; Grevsen, K. Chitosan oligosaccharide and salicylic acid up-regulate gene expression differently in relation to the biosynthesis of artemisinin in Artemisia annua L. Process Biochem. 2012, 47, 1559–1562. [Google Scholar] [CrossRef]
- Ait Barka, E.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Baque, M.A.; Shiragi, M.H.K.; Lee, E.-J.; Paek, K.-Y. Elicitor effect of chitosan and pectin on the biosynthesis of anthraquinones, phenolics and flavonoids in adventitious root suspension cultures of “Morinda citrifolia” (L.). Aust. J. Crop Sci. 2012, 6, 1349–1355. [Google Scholar] [CrossRef]
- Suarez-Fernandez, M.; Marhuenda-Egea, F.C.; Lopez-Moya, F.; Arnao, M.B.; Cabrera-Escribano, F.; Nueda, M.J.; Gunsé, B.; Lopez-Llorca, L.V. Chitosan induces plant hormones and defenses in tomato root exudates. Front. Plant Sci. 2020, 11, 572087. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Escudero, N.; Zavala-Gonzalez, E.A.; Esteve-Bruna, D.; Blázquez, M.A.; Alabadí, D.; Lopez-Llorca, L.V. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci. Rep. 2017, 7, 16813. [Google Scholar] [CrossRef] [Green Version]
- Suman, S.; Bagal, D.; Jain, D.; Singh, R.; Singh, I.K.; Singh, A. Chapter 14—Biotic stresses on plants: Reactive oxygen species generation and antioxidant mechanism. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 381–411. ISBN 978-0-323-90943-3. [Google Scholar]
- Medina, E.; Kim, S.-H.; Yun, M.; Choi, W.-G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of stress and defense in plant secondary metabolites production. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2021; pp. 151–195. ISBN 978-3-030-54027-2. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Zuk, M.; Szperlik, J.; Hnitecka, A.; Szopa, J. Temporal biosynthesis of flavone constituents in flax growth stages. Plant Physiol. Biochem. 2019, 142, 234–245. [Google Scholar] [CrossRef]
- Piasecka, A.; Kachlicki, P.; Stobiecki, M. Analytical methods for detection of plant metabolomes changes in response to biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 379. [Google Scholar] [CrossRef] [Green Version]
- Beejmohun, V.; Fliniaux, O.; Hano, C.; Pilard, S.; Grand, E.; Lesur, D.; Cailleu, D.; Lamblin, F.; Lainé, E.; Kovensky, J.; et al. Coniferin dimerisation in lignan biosynthesis in flax cells. Phytochemistry 2007, 68, 2744–2752. [Google Scholar] [CrossRef]
- Zálešák, F.; Bon, D.J.-Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef] [PubMed]
- Tashackori, H.; Sharifi, M.; Ahmadian Chashmi, N.; Safaie, N.; Behmanesh, M. Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tissue Organ Cult. PCTOC 2016, 127, 187–194. [Google Scholar] [CrossRef]
- Ziegler, J.; Schmidt, S.; Chutia, R.; Müller, J.; Böttcher, C.; Strehmel, N.; Scheel, D.; Abel, S. Non-targeted profiling of semi-polar metabolites in Arabidopsis root exudates uncovers a role for coumarin secretion and lignification during the local response to phosphate limitation. J. Exp. Bot. 2016, 67, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- An, D.; Wu, C.-H.; Wang, M.; Wang, M.; Chang, G.-N.; Chang, X.-J.; Lian, M.-L. Methyl jasmonate elicits enhancement of bioactive compound synthesis in adventitious root co-culture of Echinacea purpurea and Echinacea pallida. Vitro Cell. Dev. Biol.-Plant 2021, 58, 181–187. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-Y.; Cosma, G.; Gardner, H.; Vallyathan, V.; Castranova, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Quéro, A.; Fliniaux, O.; Elboutachfaiti, R.; Petit, E.; Guillot, X.; Hawkins, S.; Courtois, J.; Mesnard, F. β-Aminobutyric acid increases drought tolerance and reorganizes solute content and water homeostasis in flax (Linum usitatissimum). Metabolomics 2015, 11, 1363–1375. [Google Scholar] [CrossRef]
- Tocquin, P.; Corbesier, L.; Havelange, A.; Pieltain, A.; Kurtem, E.; Bernier, G.; Périlleux, C. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 2003, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. Growing plants without soil by the water-culture method. Circ. Calif. Agric. Exp. Stn. 1938, 16. [Google Scholar]
- Pontarin, N.; Molinié, R.; Mathiron, D.; Tchoumtchoua, J.; Bassard, S.; Gagneul, D.; Thiombiano, B.; Demailly, H.; Fontaine, J.-X.; Guillot, X.; et al. Age-dependent metabolic profiles unravel the metabolic relationships within and between flax Leaves (Linum usitatissimum). Metabolites 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rudaz, S. Exploring omics data from designed experiments using analysis of variance multiblock orthogonal partial least squares. Anal. Chim. Acta 2016, 920, 18–28. [Google Scholar] [CrossRef] [PubMed]
- AMOPLS. Available online: https://gitlab.unige.ch/Julien.Boccard/amopls (accessed on 17 May 2021).






| Compound Number | Family | Compounds | Part Used | Identification |
|---|---|---|---|---|
| 1 | Cyanogenic glycoside | Linamarin | shoots, roots | annotation with standard |
| 2 | Cyanogenic glycoside | Lotaustralin | shoots, roots | annotation with standard |
| 3 | Flavonoids a | Isoorientin | shoots | [22] |
| 4 | Flavonoids a | Lucenin-2 | shoots | [22] |
| 5 | Flavonoids a | Carlinoside | shoots | [22] |
| 6 | Flavonoids a | Carlinoside isomer | shoots | putative MS/MS annotation |
| 7 | Flavonoids b | Isovitexin | shoots | [22] |
| 8 | Flavonoids b | Vitexin | shoots | [22] |
| 9 | Flavonoids b | Vicenin-1 | shoots | [22] |
| 10 | Flavonoids b | Vicenin-2 | shoots | [22] |
| 11 | Flavonoids | Triticuside A | shoots | [22] |
| 12 | Flavonoids b | Schaftoside | shoots | [22] |
| 13 | Flavonoids b | Schaftoside isomer | shoots | putative MS/MS annotation |
| 14 | Hydroxycinnamic acids | Coniferin | roots | [22] |
| 15 | Hydroxycinnamic acids | HHMPG | roots | [22] |
| 16 | Hydroxycinnamic acids | 3-O-caffeoylquinic acid | shoots | annotation with standard |
| 17 | Hydroxycinnamic acids | Caffeoylquinic acid isomer | shoots | putative MS/MS annotation |
| 18 | Hydroxycinnamic acids | Caffeoylquinic acid hexoside | shoots | putative MS/MS annotation |
| 19 | Hydroxycinnamic acids | Caftaric acid | roots | annotation with standard |
| 20 | Hydroxycinnamic acids | Icariside F2 | roots | annotation with standard |
| 21 | Hydroxycinnamic acids | Chicoric acid | roots | annotation with standard |
| 22 | Lignans | PDG | shoots | [22] |
| 23 | Lignans | PMG | shoots | [22] |
| 24 | Lignans | DCG | shoots, roots | [22] |
| 25 | Lignans | LMG | shoots, roots | [22] |
| 26 | Lignans | (-)-Olivil 4′-O-beta-D-glucopyranoside | roots | annotation with standard |
| 27 | Lignans | Olivil isomer 1 | roots | putative MS/MS annotation |
| 28 | Lignans | Olivil isomer 2 | roots | putative MS/MS annotation |
| 29 | Lignans | Olivil isomer 3 | roots | putative MS/MS annotation |
| 30 | Lignans | Olivil isomer 4 | roots | putative MS/MS annotation |
| 31 | Lignans | SMG | roots | annotation with standard |
| 32 | Lignans | SMG isomer | roots | putative MS/MS annotation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elboutachfaiti, R.; Molinié, R.; Mathiron, D.; Maillot, Y.; Fontaine, J.-X.; Pilard, S.; Quéro, A.; Brasselet, C.; Dols-Lafargue, M.; Delattre, C.; et al. Secondary Metabolism Rearrangements in Linum usitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall. Molecules 2022, 27, 2372. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072372
Elboutachfaiti R, Molinié R, Mathiron D, Maillot Y, Fontaine J-X, Pilard S, Quéro A, Brasselet C, Dols-Lafargue M, Delattre C, et al. Secondary Metabolism Rearrangements in Linum usitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall. Molecules. 2022; 27(7):2372. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072372
Chicago/Turabian StyleElboutachfaiti, Redouan, Roland Molinié, David Mathiron, Yannis Maillot, Jean-Xavier Fontaine, Serge Pilard, Anthony Quéro, Clément Brasselet, Marguerite Dols-Lafargue, Cédric Delattre, and et al. 2022. "Secondary Metabolism Rearrangements in Linum usitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall" Molecules 27, no. 7: 2372. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072372