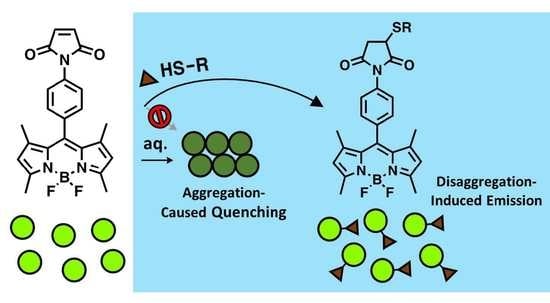
A BODIPY-Based Probe Enables Fluorogenicity via Thiol-Dependent Modulation of Fluorophore Aggregation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aggregation-Caused Quenching of p-MB in Aqueous Solution
2.2. Inhibition of Aggregation-Caused Quenching of p-MB via Conjugation with Polar Substrates
2.3. p-MB as a Fluorogenic Probe for TCEP
2.4. Rethinking the Mechanism of DIE
3. Conclusions
4. Materials and Methods
4.1. Spectroscopic Properties of p-MB in Aqueous Solution
4.2. Reaction of p-MB with Thiol Substrates in Aqueous Solution
4.3. Comparison of Reaction Kinetics with or without Preformed Aggregates of p-MB
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem.–A Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Squeo, B.M.; Pasini, M. BODIPY platform: A tunable tool for green to NIR OLEDs. Supramol. Chem. 2020, 32, 56–70. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent Progress of BODIPY Dyes With Aggregation-Induced Emission. Front. Chem. 2019, 7, 712. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem 2020, 4, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Urano, Y.; Shoda, T.; Kojima, H.; Nagano, T. A Thiol-Reactive Fluorescence Probe Based on Donor-Excited Photoinduced Electron Transfer: Key Role of Ortho Substitution. Org. Lett. 2007, 9, 3375–3377. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Zhai, D.; Xu, W.; Zhang, L.; Chang, Y.-T. The role of ‘disaggregation’ in optical probe development. Chem. Soc. Rev. 2014, 43, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, K.; Ishida, Y.; Takaoka, Y.; Miyagawa, M.; Tsukiji, S.; Hamachi, I. Disassembly-Driven Turn-On Fluorescent Nanoprobes for Selective Protein Detection. J. Am. Chem. Soc. 2010, 132, 7291–7293. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Agrawalla, B.K.; Eng, P.S.F.; Lee, S.-C.; Xu, W.; Chang, Y.-T. Development of a fluorescent sensor for an illicit date rape drug–GBL. Chem. Commun. 2013, 49, 6170–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W. Make Caffeine Visible: A Fluorescent Caffeine ‘Traffic Light’ Detector. Sci. Rep. 2013, 3, 2255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, H.; Luo, Z.; Chang, Y.-T.; Zhang, L. Development of a disaggregation-induced emission probe for the detection of RecA inteins from Mycobacterium tuberculosis. Chem. Commun. 2016, 52, 9086–9088. [Google Scholar] [CrossRef]
- Gambardella, G.; Cattani, G.; Bocedi, A.; Ricci, G. New Factors Enhancing the Reactivity of Cysteines in Molten Globule-Like Structures. Int. J. Mol. Sci. 2020, 21, 6949. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Getz, E.B.; Xiao, M.; Chakrabarty, T.; Cooke, R.; Selvin, P.R. A Comparison between the Sulfhydryl Reductants Tris(2-carboxyethyl)phosphine and Dithiothreitol for Use in Protein Biochemistry. Anal. Biochem. 1999, 273, 73–80. [Google Scholar] [CrossRef]
- Tyagarajan, K.; Pretzer, E.; Wiktorowicz, J.E. Thiol-reactive dyes for fluorescence labeling of proteomic samples. Electrophoresis 2003, 24, 2348–2358. [Google Scholar] [CrossRef]
- Henkel, M.; Röckendorf, N.; Frey, A. Selective and Efficient Cysteine Conjugation by Maleimides in the Presence of Phosphine Reductants. Bioconjugate Chem. 2016, 27, 2260–2265. [Google Scholar] [CrossRef]
- Kantner, T.; Alkhawaja, B.; Watts, A.G. In Situ Quenching of Trialkylphosphine Reducing Agents Using Water-Soluble PEG-Azides Improves Maleimide Conjugation to Proteins. ACS Omega 2017, 2, 5785–5791. [Google Scholar] [CrossRef]
- Saito, F.; Noda, H.; Bode, J.W. Critical Evaluation and Rate Constants of Chemoselective Ligation Reactions for Stoichiometric Conjugations in Water. ACS Chem. Biol. 2015, 10, 1026–1033. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chio, T.I.; Grimaldi, A.J.; Radford, T.I.; Bane, S.L. A BODIPY-Based Probe Enables Fluorogenicity via Thiol-Dependent Modulation of Fluorophore Aggregation. Molecules 2022, 27, 2455. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082455
Chio TI, Grimaldi AJ, Radford TI, Bane SL. A BODIPY-Based Probe Enables Fluorogenicity via Thiol-Dependent Modulation of Fluorophore Aggregation. Molecules. 2022; 27(8):2455. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082455
Chicago/Turabian StyleChio, Tak Ian, Akiva J. Grimaldi, Thomas I. Radford, and Susan L. Bane. 2022. "A BODIPY-Based Probe Enables Fluorogenicity via Thiol-Dependent Modulation of Fluorophore Aggregation" Molecules 27, no. 8: 2455. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082455