Herbicide and Cytogenotoxic Activity of Inclusion Complexes of Psidium gaudichaudianum Leaf Essential Oil and β-Caryophyllene on 2-Hydroxypropyl-β-cyclodextrin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Inclusion Complexes and Characterizations
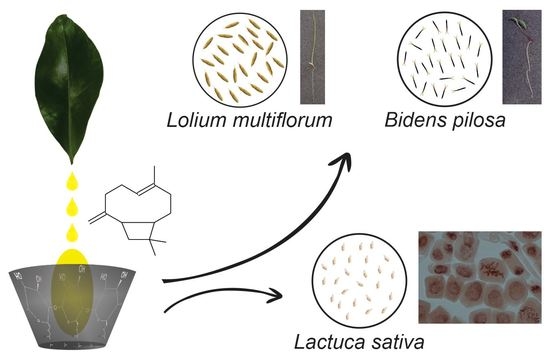
2.2. Herbicidal Activity
2.3. Cytogenotoxic Activity
3. Materials and Methods
3.1. Standards and Reagents
3.2. Collection and Extraction of Essential Oil from the Leaves of P. gaudichaudianum
3.3. Determination of Absolute Density and Yield of Essential Oil Extraction
3.4. Essential Oil Chromatographic Profile
3.5. Preparation of the Inclusion Complex
3.6. Characterization of the Inclusion Complex
3.6.1. Analytical Curve
3.6.2. Thermogravimetric Analysis
3.6.3. Fourier Transform Infrared Spectroscopy
3.7. Herbicidal Activity Evaluation
3.8. Cytotoxicity Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaur, P.; Gupta, S.; Kaur, K.; Kaur, N.; Kumar, R.; Bhullar, M.S. Nanoemulsion of Foeniculum Vulgare Essential Oil: A Propitious Striver against Weeds of Triticum Aestivum. Ind. Crops Prod. 2021, 168, 113601. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global Threat to Agriculture from Invasive Species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- El-Alam, I.; Raveau, R.; Fontaine, J.; Verdin, A.; Laruelle, F.; Fourmentin, S.; Chahine, R.; Makhlouf, H.; Lounès-Hadj Sahraoui, A. Antifungal and Phytotoxic Activities of Essential Oils: In Vitro Assays and Their Potential Use in Crop Protection. Agronomy 2020, 10, 825. [Google Scholar] [CrossRef]
- Xie, B.; Han, G.; Qiao, P.; Mei, B.; Wang, Q.; Zhou, Y.; Zhang, A.; Song, W.; Guan, B. Effects of Mechanical and Chemical Control on Invasive Spartina alterniflora in the Yellow River Delta, China. PeerJ 2019, 7, e7655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daba, A.; Berecha, G.; Tadesse, M. Herbicidal Effects of Essential Oils from Selected Plant Species against Common Coffee (Coffea arabica L.) Weed Species. Acta Physiol. Plant. 2021, 43, 162. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Zermane, N.; Abdelkrim, H. Chemical Composition and Herbicidal Activity of Essential Oils from Two Labiatae Species from Algeria. J. Essent. Oil Res. 2019, 31, 335–346. [Google Scholar] [CrossRef]
- Ibáñez, M.; Blázquez, M. Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, C.G.; de Paula Amaral, L.; Rolim, J.M.; Longhi, S.J.; de Oliveira Machado, S.L.; Heinzmann, B.M. Essential Oil of the Brazilian Native Species Hesperozygis ringens: A Potential Alternative to Control Weeds. J. Essent. Oil Bear. Plants 2017, 20, 701–711. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, R.; Huang, J.; Huang, X.; Shi, G.; Li, Y.; Yin, Y.; Chen, Z. Health Effect of Agricultural Pesticide Use in China: Implications for the Development of GM Crops. Sci. Rep. 2016, 6, 34918. [Google Scholar] [CrossRef]
- Maes, C.; Meersmans, J.; Lins, L.; Bouquillon, S.; Fauconnier, M.-L. Essential Oil-Based Bioherbicides: Human Health Risks Analysis. Int. J. Mol. Sci. 2021, 22, 9396. [Google Scholar] [CrossRef]
- Hojjati, M.; Barzegar, H. Chemical Composition and Biological Activities of Lemon (Citrus limon) Leaf Essential Oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Zandi-Sohani, N.; Rajabpour, A.; Yarahmadi, F.; Ramezani, L. Sensitivity of Bemisia tabaci (Hemiptera: Aleyrodidae) and the Generalist Predator Orius Albidipennis (Hemiptera: Anthocoridae) to Vapors of Essential Oils. J. Entomol. Sci. 2018, 53, 493–502. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus Citriodora, Lavandula Angustifolia, and Pinus Sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.C.S.; Medeiros Filho, S.; Manoel Neto, A.; Brito, R.C.; Araujo, R.C. Allelopathic Effect of Essential Oils of Medicinal Plants in Bidens pilosa L. Rev. Bras. Plantas Med. 2014, 16, 731–736. [Google Scholar] [CrossRef] [Green Version]
- e Silva, R.C.; da Costa, J.S.; de Figueiredo, R.O.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, L.C.; de Souza Santos, E.; de Oliveira Bernardes, C.; da Silva Ferreira, M.F.; Ferreira, A.; Tuler, A.C.; Carvalho, J.A.M.; Pinheiro, P.F.; Praça-Fontes, M.M. Phytochemical Analysis and Effect of the Essential Oil of Psidium L. Species on the Initial Development and Mitotic Activity of Plants. Environ. Sci. Pollut. Res. 2019, 26, 26216–26228. [Google Scholar] [CrossRef]
- Arthur, G.D.; Naidoo, K.K.; Coopoosamy, R.M. Bidens pilosa L.: Agricultural and Pharmaceutical Importance. J. Med. Plants Res. 2012, 6, 3282–3287. [Google Scholar] [CrossRef]
- Firestone, J.L.; Jasieniuk, M. Seed Production Is Reduced by Small Population Size in Natural Populations of the Invasive Grass Lolium Multiflorum. Biol. Invasions 2012, 14, 2519–2529. [Google Scholar] [CrossRef]
- Mossa, A.-T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-Pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef] [Green Version]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Silveira, G.L.; Lima, M.G.F.; dos Reis, G.B.; Palmieri, M.J.; Andrade-Vieria, L.F. Toxic Effects of Environmental Pollutants: Comparative Investigation Using Allium cepa L. and Lactuca sativa L. Chemosphere 2017, 178, 359–367. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Santiago, J.; das Graças Cardoso, M.; da Cruz, F.A.; Palmieri, M.J.; Souza, R.; Soares, L.I.; de Campos, J.M.S.; Andrade-Vieira, L.F. Cytogenotoxic Effect of Essential Oil from Backhousia citriodora L. (Myrtaceae) on Meristematic Cells of Lactuca sativa L. S. Afr. J. Bot. 2017, 112, 515–520. [Google Scholar] [CrossRef]
- Mendes, L.A.; Silva, R.R.A.; de Fátima Ferreira Soares, N.; Martins, G.F.; Teixeira, R.R.; da Silva Ferreira, M.F.; Moreira, R.P.L. Development of Inclusion Complexes of 2-Hydroxypropyl-β-Cyclodextrin with Psidium Guajava L. Essential Oil by Freeze-Drying and Kneading Methods for Application as Aedes Aegypti L. Larvicide. Nat. Prod. Res. 2022, 37, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Silva, R.C.; da Silva, J.K.R.; Suemitsu, C.; Mourão, R.H.V.; Maia, J.G.S. Chemical Variability in the Essential Oil of Leaves of Araçá (Psidium guineense Sw.), with Occurrence in the Amazon. Chem. Cent. J. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Savoldi, T.L.; Glamoćlija, J.; Soković, M.; Gonçalves, J.E.; Ruiz, S.P.; Linde, G.A.; Gazim, Z.C.; Colauto, N.B. Antimicrobial Activity of Essential Oil from Psidium Cattleianum Afzel. Ex Sabine Leaves. BLACPMA 2020, 19, 614–627. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; El-Ghorab, A.H.; Hussein, A.M.S.; El-Massry, K.F.; Lotfy, S.N.; Sayed Ahmed, M.Y.; Soliman, T.N. Correlation between Chemical Composition and Radical Scavenging Activity of 10 Commercial Essential Oils: Impact of Microencapsulation on Functional Properties of Essential Oils. Arab. J. Chem. 2020, 13, 6815–6827. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and Antioxidant Activities of Carvacrol Microencapsulated in Hydroxypropyl-Beta-Cyclodextrin. LWT—Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Ozdemir, N.; Hill, L.E.; Gomes, C.L. Synthesis and Characterization of Nano-Encapsulated Black Pepper Oleoresin Using Hydroxypropyl Beta-Cyclodextrin for Antioxidant and Antimicrobial Applications: Black Pepper Oleoresin Nanoparticles. J. Food Sci. 2013, 78, N1913–N1920. [Google Scholar] [CrossRef]
- Babaoglu, H.C.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of Clove Essential Oil in Hydroxypropyl Beta-Cyclodextrin for Characterization, Controlled Release, and Antioxidant Activity. J. Food Process. Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- Poonphatanapricha, T.; Katanyutanon, S.; Jitapunkul, K.; Lawtrakul, L.; Toochinda, P. The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins. Sci. Pharm. 2021, 89, 42. [Google Scholar] [CrossRef]
- Marreto, R.N.; Almeida, E.E.C.V.; Alves, P.B.; Niculau, E.S.; Nunes, R.S.; Matos, C.R.S.; Araújo, A.A.S. Thermal Analysis and Gas Chromatography Coupled Mass Spectrometry Analyses of Hydroxypropyl-β-Cyclodextrin Inclusion Complex Containing Lippia Gracilis Essential Oil. Thermochim. Acta 2008, 475, 53–58. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of Garlic Oil by Β-cyclodextrin as a Thermal Protection Method for Antibacterial Action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Qin, Y.; Zhang, K.; Bi, Y.; Kong, F. Inclusion Complex of Exocarpium Citri Grandis Essential Oil with Β-Cyclodextrin: Characterization, Stability, and Antioxidant Activity. J. Food Sci. 2019, 84, 1592–1599. [Google Scholar] [CrossRef]
- Gao, S.; Bie, C.; Ji, Q.; Ling, H.; Li, C.; Fu, Y.; Zhao, L.; Ye, F. Preparation and Characterization of Cyanazine–Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex. RSC Adv. 2019, 9, 26109–26115. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Prabu, S.; Swaminathan, M.; Sivakumar, K.; Rajamohan, R. Preparation, Characterization and Molecular Modeling Studies of the Inclusion Complex of Caffeine with Beta-Cyclodextrin. J. Mol. Struct. 2015, 1099, 616–624. [Google Scholar] [CrossRef]
- Barbosa, L.C.d.A. Espectroscopia No Infravermelho, 1st ed.; UFV: Viçosa, Brazil, 2007; ISBN 978-85-7269-280-9. [Google Scholar]
- Gao, S.; Liu, Y.; Jiang, J.; Ji, Q.; Fu, Y.; Zhao, L.; Li, C.; Ye, F. Physicochemical Properties and Fungicidal Activity of Inclusion Complexes of Fungicide Chlorothalonil with β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and Antimicrobial Properties of Encapsulated Guava Leaf Oil in Hydroxypropyl-Beta-Cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Pinheiro, J.; Tavares, E.; Silva, S.; Félix Silva, J.; Carvalho, Y.; Ferreira, M.; Araújo, A.; Barbosa, E.; Fernandes Pedrosa, M.; Soares, L.; et al. Inclusion Complexes of Copaiba (Copaifera Multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. IJMS 2017, 18, 2388. [Google Scholar] [CrossRef] [Green Version]
- Pires, F.Q.; Pinho, L.A.; Freire, D.O.; Silva, I.C.R.; Sa-Barreto, L.L.; Cardozo-Filho, L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Thermal Analysis Used to Guide the Production of Thymol and Lippia Origanoides Essential Oil Inclusion Complexes with Cyclodextrin. J. Therm. Anal. Calorim. 2019, 137, 543–553. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.-D.; Le, Q.-T. Biological Activities and Chemical Constituents of Essential Oils from Piper Cubeba Bojer and Piper Nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, M.; Blázquez, M. Ginger and Turmeric Essential Oils for Weed Control and Food Crop Protection. Plants 2019, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Yuet Ping, K.; Darah, I.; Yusuf, U.K.; Yeng, C.; Sasidharan, S. Genotoxicity of Euphorbia Hirta: An Allium Cepa Assay. Molecules 2012, 17, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Harashima, H.; Schnittger, A. The Integration of Cell Division, Growth and Differentiation. Curr. Opin. Plant Biol. 2010, 13, 66–74. [Google Scholar] [CrossRef]
- Gonçalves, M.d.M.C.; Lopes, A.C.d.A.; Gomes, R.L.F.; de Melo, W.J.; Araujo, A.S.F.; Pinheiro, J.B.; Marin-Morales, M.A. Phytotoxicity and Cytogenotoxicity of Composted Tannery Sludge. Environ. Sci. Pollut. Res. 2020, 27, 34495–34502. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.; Mantovani, M.S.; Marin-Morales, M.A. Analysis of the Genotoxic Potential of Low Concentrations of Malathion on the Allium Cepa Cells and Rat Hepatoma Tissue Culture. J. Environ. Sci. 2015, 36, 102–111. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium Cepa Test in Environmental Monitoring: A Review on Its Application. Mutat. Res./Rev. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Fernandes, T.C.C.; Mazzeo, D.E.C.; Marin-Morales, M.A. Mechanism of Micronuclei Formation in Polyploidizated Cells of Allium Cepa Exposed to Trifluralin Herbicide. Pestic. Biochem. Physiol. 2007, 88, 252–259. [Google Scholar] [CrossRef]
- Fernandes, T.C.C.; Mazzeo, D.E.C.; Marin-Morales, M.A. Origin of Nuclear and Chromosomal Alterations Derived from the Action of an Aneugenic Agent—Trifluralin Herbicide. Ecotoxicol. Environ. Saf. 2009, 72, 1680–1686. [Google Scholar] [CrossRef]
- Freitas, A.S.; Fontes Cunha, I.M.; Andrade-Vieira, L.F.; Techio, V.H. Effect of SPL (Spent Pot Liner) and Its Main Components on Root Growth, Mitotic Activity and Phosphorylation of Histone H3 in Lactuca sativa L. Ecotoxicol. Environ. Saf. 2016, 124, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.J.; Barroso, A.R.; Andrade-Vieira, L.F.; Monteiro, M.C.; Soares, A.M.; Cesar, P.H.S.; Braga, M.A.; Trento, M.V.C.; Marcussi, S.; Davide, L.C. Polybia occidentalis and Polybia fastidiosa Venom: A Cytogenotoxic Approach of Effects on Human and Vegetal Cells. Drug Chem. Toxicol. 2021, 44, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Vieira, L.F.; Gedraite, L.S.; Campos, J.M.S.; Davide, L.C. Spent Pot Liner (SPL) Induced DNA Damage and Nuclear Alterations in Root Tip Cells of Allium Cepa as a Consequence of Programmed Cell Death. Ecotoxicol. Environ. Saf. 2011, 74, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.F.; Campos, J.M.S.; Davide, L.C. Cytogenetic Alterations Induced by SPL (Spent Potliners) in Meristematic Cells of Plant Bioassays. Ecotoxicol. Environ. Saf. 2008, 71, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic Effects of Volatile Monoterpenoids Produced by Salvia Leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the Root Apical Meristem of Brassica Campestris Seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Sousa, V.D. Farmacopeia Brasileira; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2019; Volume 2.
- Mendes, L.A.; Martins, G.F.; Valbon, W.R.; da Silva de Souza, T.; Menini, L.; Ferreira, A.; da Silva Ferreira, M.F. Larvicidal Effect of Essential Oils from Brazilian Cultivars of Guava on Aedes aegypti L. Ind. Crops Prod. 2017, 108, 684–689. [Google Scholar] [CrossRef]
- da Silva de Souza, T.; da Silva Ferreira, M.F.; Menini, L.; de Lima Souza, J.R.C.; Parreira, L.A.; Cecon, P.R.; Ferreira, A. Essential Oil of Psidium Guajava: Influence of Genotypes and Environment. Sci. Hortic. 2017, 216, 38–44. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1-932633-21-9. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 27 May 2020).
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry (accessed on 27 May 2020).
- Mendes, L.A.; Silva, R.R.A.; Oliveira, E.E.D.; Corrêa, M.J.M.; Marques, C.S.; Ferreira, M.F.D.S.; Teixeira, R.R.; Moreira, R.P.L. Optimization of Inclusion Complex’s Preparation of Psidium Cattleyanum S. Essential Oil and 2-Hydroxypropyl-β-Cyclodextrin by Central Composite Design for Application as Larvicide in Aedes aegypti L. Ind. Crops Prod. 2023, 194, 116333. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing Essential Oils (Trans-Cinnamaldehyde, Eugenol, Cinnamon Bark, and Clove Bud Extracts) for Antimicrobial Delivery Applications. LWT—Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Tigre, R.C.; Silva, N.H.; Santos, M.G.; Honda, N.K.; Falcão, E.P.S.; Pereira, E.C. Allelopathic and Bioherbicidal Potential of Cladonia Verticillaris on the Germination and Growth of Lactuca sativa. Ecotoxicol. Environ. Saf. 2012, 84, 125–132. [Google Scholar] [CrossRef]
- Singh, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S. Chemical Characterization, Phytotoxic, and Cytotoxic Activities of Essential Oil of Mentha Longifolia. Environ. Sci. Pollut. Res. 2020, 27, 13512–13523. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Sommaggio, L.R.D.; Marin-Morales, M.A. Phyto-Genotoxicity Assessment of Different Associations between Sludges from Water and Sewage Treatment Plants, before and after the Bioremediation Process. Environ. Sci. Pollut. Res. 2022, 29, 40029–40040. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]












| n | Compound a | tretention b | Arelative (%) c | Molar Mass (g mol−1) | Terpene Classification d |
|---|---|---|---|---|---|
| 1 | α-pinene | 8.549 | 9.2 | 136.24 | HM |
| 2 | limonene | 12.833 | 9.8 | 136.24 | HM |
| 3 | 1,8-cineole | 12.833 | 4.3 | 154.25 | OM |
| 4 | γ-terpinene | 14.149 | 2.9 | 136.24 | HM |
| 5 | β-caryophyllene | 30.646 | 53.3 | 204.36 | HS |
| 6 | α-humulene | 32.003 | 12.0 | 204.36 | HS |
| 7 | caryophyllene oxide | 37.519 | 5.0 | 220.36 | OS |
| Total identified | 96.5 |
| Sample | Δm1 (%) a 30–110 °C | Δm2 (%) b 110–200 °C | Δm3 (%) c 200–400 °C |
|---|---|---|---|
| HPβCD | 6.4 | 0.1 | 81.4 |
| GAU | 17.4 | 77.9 | - |
| β-CAR | 5.3 | 94.4 | - |
| GAU IC | 7.9 | 1.6 | 76.5 |
| β-CAR IC | 9.8 | 0.4 | 75.9 |
| Source of Variation | df | Germination (%) | Root Growth (mm) | Aerial Growth (mm) |
|---|---|---|---|---|
| Invasive plant species (A) | 1 | 345.2801 *** | 160.437 *** | 4.9259 * |
| Treatment (B) | 3 | 63.6608 *** | 158.2757 *** | 219.206 *** |
| Concentration (C) | 4 | 26.706 *** | 8.5239 *** | 26.9576 *** |
| A X B | 3 | 27.9444 *** | 58.5316 *** | 5.4023 ** |
| A X C | 4 | 3.6755 ** | 1.5868 | 2.1215 |
| B X C | 12 | 13.4208 *** | 10.8153 *** | 24.6083 *** |
| A X B X C | 12 | 7.519 *** | 4.804 *** | 7.7438 *** |
| Coefficient of variation | 20.53% | 23.69% | 23.37% |
| Concentrations (µg mL−1) | Treatments | Germination (G) | Root Growth (RG) | Aerial Growth (AG) | |||
|---|---|---|---|---|---|---|---|
| B. pilosa | L. multiflorum | B. pilosa | L. multiflorum | B. pilosa | L. multiflorum | ||
| 3000 | GAU EO | 3.2 ± 3.3 Bc | 23.2 ± 15.8 Ab | 2.3 ± 2.6 Bc | 9.3 ± 5.7 Ab | 0.0 ± 0.0 Ad | 5.1 ± 3.0 Ac |
| GAU IC | 52.8 ± 18.8 Ab | 19.2 ± 4.4 Bb | 15.3 ± 2.4 Ab | 13.2 ± 5.8 Aab | 14.3 ± 0.7 Ac | 13.0 ± 7.0 Ab | |
| β-CAR | 86.4 ± 10.4 Aa | 44.8 ± 15.6 Ba | 20.3 ± 3.8 Ab | 19.8 ± 6.2 Aa | 22.3 ± 5.1 Ab | 21.9 ± 6.8 Aa | |
| β-CAR IC | 82.4 ± 6.1 Aa | 28.8 ± 11.8 Bab | 40.3 ± 1.8 Aa | 13.2 ± 3.9 Bab | 31.1 ± 5.3 Aa | 13.6 ± 4.1 Bb | |
| 1500 | GAU EO | 4.0 ± 6.9 Bb | 30.4 ± 14.3 Ab | 1.8 ± 2.5 Bc | 8.1 ± 1.6 Ab | 0.0 ± 0.0 Ac | 5.3 ± 1.7 Ac |
| GAU IC | 79.2 ± 7.7 Aa | 35.2 ± 7.7 Bab | 19.2 ± 1.1 Ab | 17.1 ± 6.3 Aa | 16.2 ± 4.3 Bb | 22.1 ± 4.5 Aab | |
| β-CAR | 92.0 ± 5.7 Aa | 49.6 ± 8.8 Ba | 24.1 ± 3.6 Ab | 16.6 ± 5.4 Ba | 26.4 ± 5.7 Aa | 16.6 ± 7.3 Bb | |
| β-CAR IC | 80.0 ± 9.4 Aa | 44.8 ± 15.6 Bab | 41.6 ± 4.2 Aa | 15.9 ± 3.0 Ba | 28.3 ± 7.9 Aa | 27.4 ± 4.0 Aa | |
| 750 | GAU EO | 44.0 ± 25.3 Ab | 29.6 ± 4.6 Ab | 6.1 ± 3.8 Ac | 9.8 ± 3.0 Ab | 4.4 ± 4.1 Ac | 5.5 ± 1.1 Ac |
| GAU IC | 79.2 ± 12.5 Aa | 52 ± 11.0 Ba | 24.4 ± 2.4 Ab | 18.5 ± 5.9 Ba | 19.2 ± 1.2 Ab | 12.6 ± 3.3 Bb | |
| β-CAR | 92.8 ± 8.2 Aa | 48.8 ± 12.1 Ba | 27.9 ± 3.4 Ab | 20.7 ± 3.3 Ba | 29.4 ± 4.4 Aa | 24.8 ± 3.2 Aa | |
| β-CAR IC | 88.0 ± 4.9 Aa | 53.6 ± 14.6 Ba | 35.4 ± 3.3 Aa | 17.5 ± 5.9 Ba | 31.4 ± 4.6 Aa | 27.7 ± 3.3 Aa | |
| 375 | GAU EO | 76.0 ± 7.5 Aa | 45.6 ± 6.7 Ba | 12.2 ± 3.8 Ac | 12.4 ± 6.2 Ab | 8.4 ± 2.9 Ab | 9.9 ± 2.3 Ab |
| GAU IC | 81.6 ± 12.2 Aa | 50.4 ± 14.6 Ba | 27.0 ± 0.7 Ab | 17.7 ± 6.2 Bab | 26.5 ± 3.3 Aa | 16.4 ± 7.0 Bb | |
| β-CAR | 83.2 ± 19.1 Aa | 0.0 ± 0.0 Bb | 13.0 ± 3.2 Ac | 0.0 ± 0.0 Bc | 0.0 ± 0.0 Ab | 0.0 ± 0.0 Ac | |
| β-CAR IC | 87.2 ± 8.7 Aa | 62.4 ± 9.2 Ba | 35.4 ± 3.3 Aa | 24.1 ± 5.5 Ba | 29.4 ± 6.4 Ba | 35.5 ± 3.4 Aa | |
| 187.5 | GAU EO | 77.6 ± 8.8 Aa | 42.4 ± 18.5 Ba | 16.4 ± 3.8 Ac | 14.9 ± 6.6 Abc | 14.9 ± 3.4 Ab | 14.2 ± 4.6 Ab |
| GAU IC | 84.0 ± 11.7 Aa | 52.8 ± 11.1 Ba | 27.0 ± 2.0 Ab | 8.6 ± 1.8 Bc | 25.9 ± 5.0 Aa | 16.9 ± 4.5 Bb | |
| β-CAR | 84.8 ± 10.4 Aa | 53.6 ± 6.7 Ba | 26.6 ± 5.9 Ab | 18.9 ± 5.8 Bab | 27.7 ± 3.6 Aa | 29.7 ± 6.4 Aa | |
| β-CAR IC | 80.0 ± 13.3 Aa | 60.0 ± 7.5 Ba | 37.1 ± 4.9 Aa | 22.3 ± 9.7 Ba | 26.1 ± 2.8 Ba | 36.6 ± 1.5 Aa | |
| Treatment | Concentration (µg mL−1) | Mitotic Index (%) | Genotoxicity (%) | Mutagenicity (%) |
|---|---|---|---|---|
| NC | - | 8.16 ± 1.08 a | 2.14 ± 0.32 a | 0.00 ± 0.00 a |
| PC | - | 8.57 ± 0.62 b | 2.88 ± 0.25 b | 0.00 ± 0.00 b |
| GAU EO | 187.5 | 8.70 ± 0.78 ab | 2.46 ± 0.71 ab | 0.00 ± 0.00 ab |
| 375 | 8.96 ± 1.09 ab | 2.24 ± 0.39 ab | 0.02 ± 0.04 ab | |
| 750 | 9.22 ± 1.29 ab | 2.36 ± 0.50 ab | 0.00 ± 0.00 ab | |
| 1500 | 8.92 ± 0.57 ab | 2.38 ± 0.29 ab | 0.04 ± 0.05 ab | |
| 3000 | 7.88 ± 1.13 ab | 2.30 ± 0.51 ab | 0.02 ± 0.04 ab | |
| β-CAR | 187.5 | 8.64 ± 0.84 ab | 2.16 ± 0.55 ab | 0.00 ± 0.00 ab |
| 375 | 4.82 ± 1.30 | 2.16 ± 0.46 ab | 0.04 ± 0.05 ab | |
| 750 | 7.2 ± 0.37 ab | 2.22 ± 0.58 ab | 0.00 ± 0.00 ab | |
| 1500 | 7.22 ± 1.18 ab | 1.96 ± 0.42 a | 0.00 ± 0.00 ab | |
| 3000 | 7.54 ± 1.24 ab | 1.98 ± 0.36 a | 0.00 ± 0.00 ab |
| Treatment | Concentration (µg mL−1) | Mitotic Index (%) | Genotoxicity (%) | Mutagenicity (%) |
|---|---|---|---|---|
| NC | - | 9.36 ± 0.62 a | 1.96 ± 0.38 a | 0.00 ± 0.00 a |
| PC | - | 8.57 ± 0.62 b | 2.88 ± 0.25 b | 0.00 ± 0.00 b |
| GAU IC | 187.5 | 8.78 ± 0.61 ab | 2.02 ± 0.30 a | 0.00 ± 0.00 ab |
| 375 | 9.10 ± 0.16 ab | 1.88 ± 0.38 a | 0.04 ± 0.05 ab | |
| 750 | 7.90 ± 0.80 b | 1.48 ± 0.51 a | 0.00 ± 0.00 ab | |
| 1500 | 7.86 ± 0.82 b | 1.72 ± 0.04 a | 0.00 ± 0.00 ab | |
| 3000 | 7.52 ± 0.49 b | 1.52 ± 0.47 a | 0.04 ± 0.05 ab | |
| β-CAR IC | 187.5 | 7.80 ± 0.69 b | 1.48 ± 0.38 a | 0.00 ± 0.00 ab |
| 375 | 8.54 ± 0.49 ab | 1.82 ± 0.29 a | 0.02 ± 0.04 ab | |
| 750 | 8.80 ± 1.19 ab | 1.82 ± 0.21 a | 0.00 ± 0.00 ab | |
| 1500 | 8.58 ± 0.44 ab | 2.54 ± 0.57 ab | 0.00 ± 0.00 ab | |
| 3000 | 8.28 ± 1.12 ab | 2.76 ± 0.36 b | 0.00 ± 0.00 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, L.A.; Vasconcelos, L.C.; Fontes, M.M.P.; Martins, G.S.; Bergamin, A.d.S.; Silva, M.A.; Silva, R.R.A.; Oliveira, T.V.d.; Souza, V.G.L.; Ferreira, M.F.d.S.; et al. Herbicide and Cytogenotoxic Activity of Inclusion Complexes of Psidium gaudichaudianum Leaf Essential Oil and β-Caryophyllene on 2-Hydroxypropyl-β-cyclodextrin. Molecules 2023, 28, 5909. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28155909
Mendes LA, Vasconcelos LC, Fontes MMP, Martins GS, Bergamin AdS, Silva MA, Silva RRA, Oliveira TVd, Souza VGL, Ferreira MFdS, et al. Herbicide and Cytogenotoxic Activity of Inclusion Complexes of Psidium gaudichaudianum Leaf Essential Oil and β-Caryophyllene on 2-Hydroxypropyl-β-cyclodextrin. Molecules. 2023; 28(15):5909. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28155909
Chicago/Turabian StyleMendes, Luiza Alves, Loren Cristina Vasconcelos, Milene Miranda Praça Fontes, Geisiele Silva Martins, Aline dos Santos Bergamin, Matheus Alves Silva, Rafael Resende Assis Silva, Taíla Veloso de Oliveira, Victor Gomes Lauriano Souza, Marcia Flores da Silva Ferreira, and et al. 2023. "Herbicide and Cytogenotoxic Activity of Inclusion Complexes of Psidium gaudichaudianum Leaf Essential Oil and β-Caryophyllene on 2-Hydroxypropyl-β-cyclodextrin" Molecules 28, no. 15: 5909. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28155909